J Korean Neurosurg Soc.
2021 May;64(3):346-358. 10.3340/jkns.2020.0362.
Overview of Secondary Neurulation
- Affiliations
-
- 1Laboratoire de Biologie du développement, Sorbonne Université, Paris, France
- KMID: 2515490
- DOI: http://doi.org/10.3340/jkns.2020.0362
Abstract
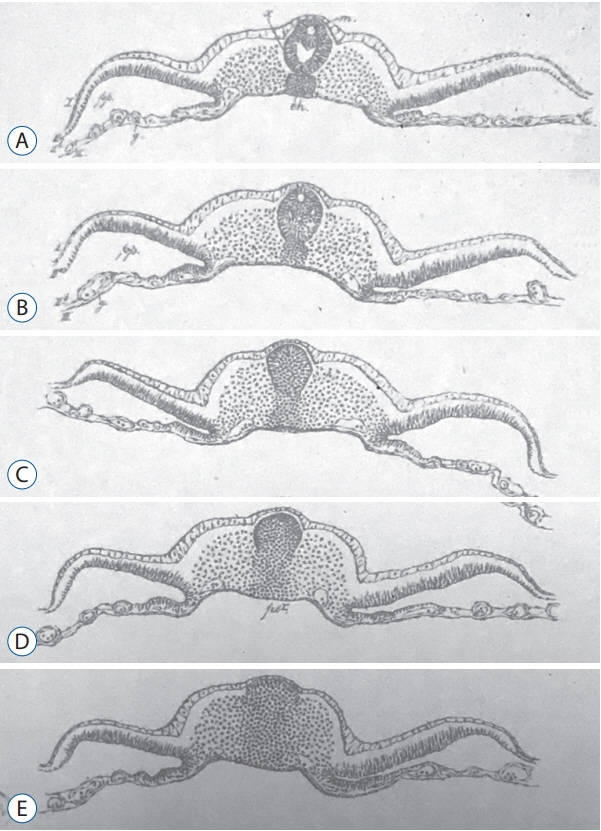
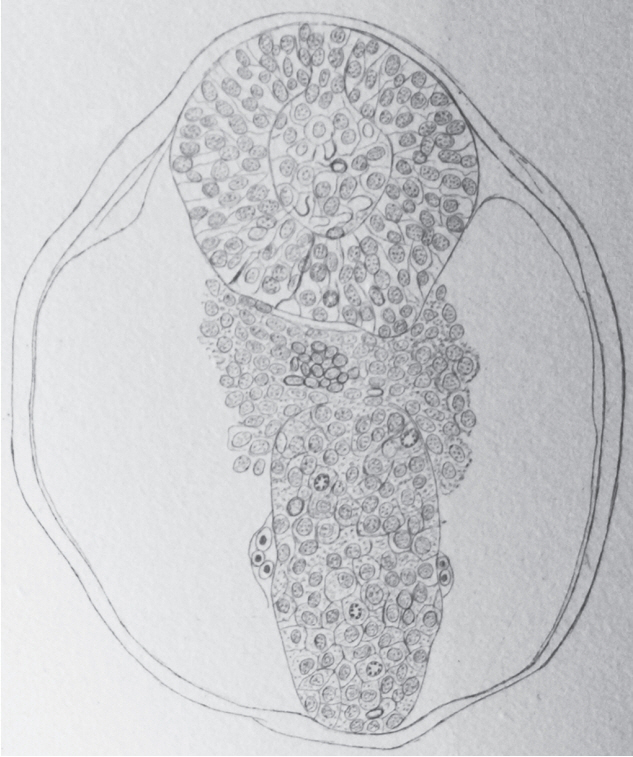
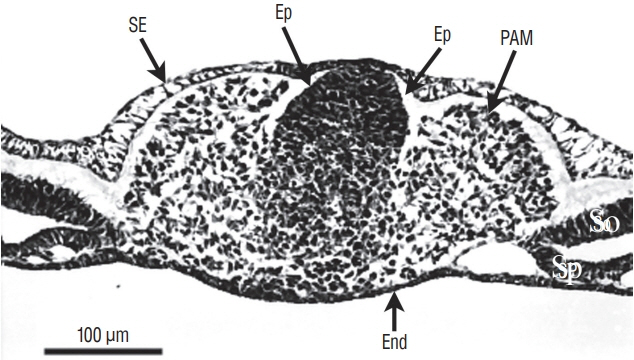
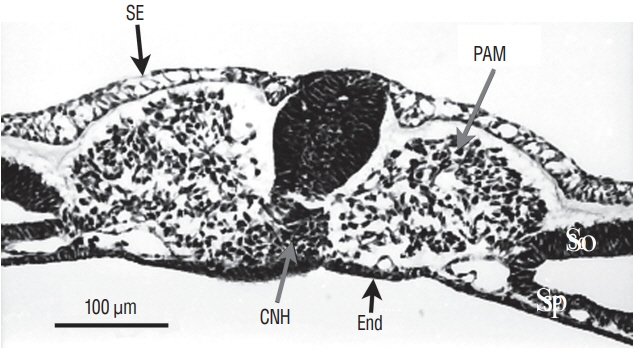
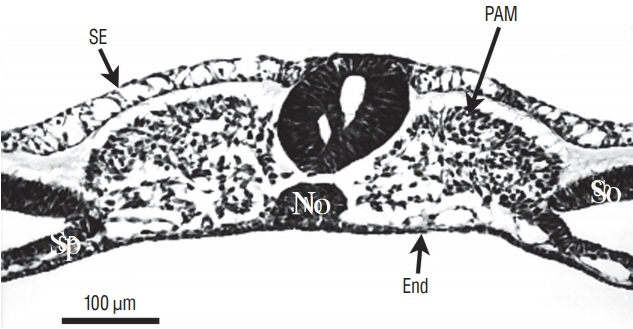
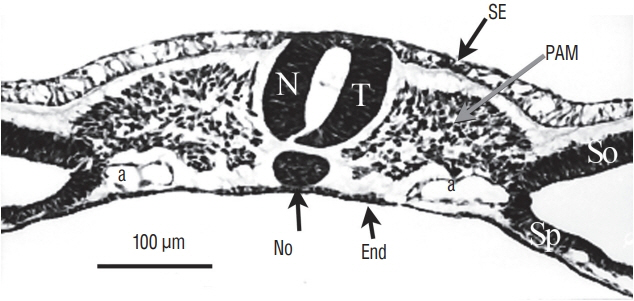
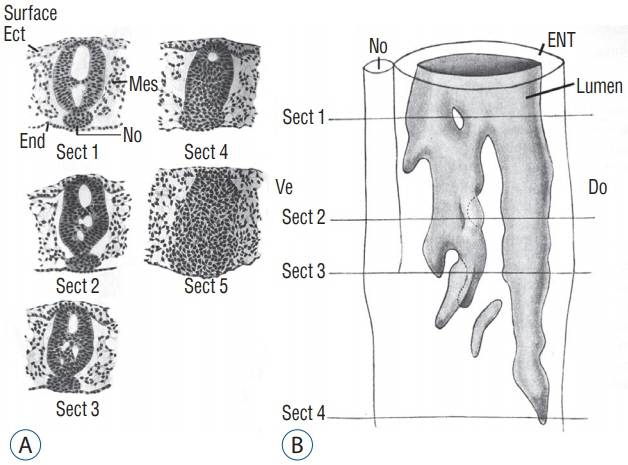
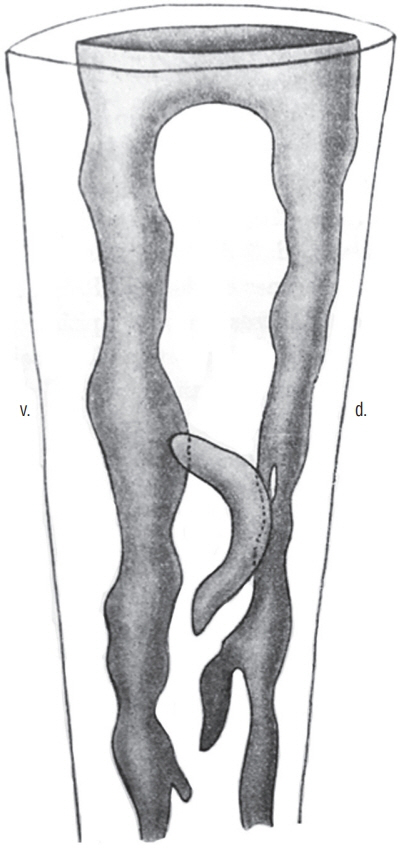
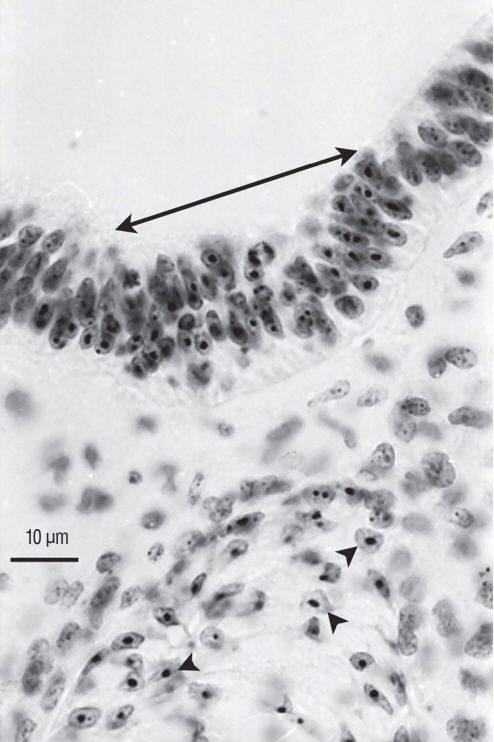
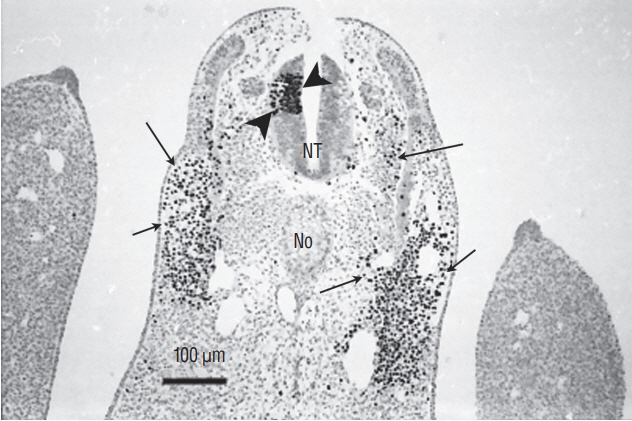
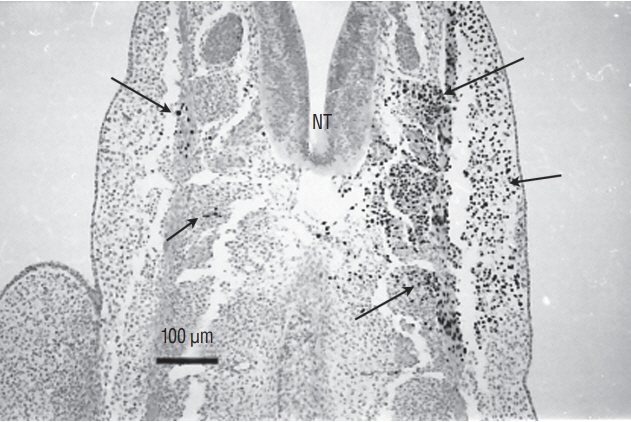
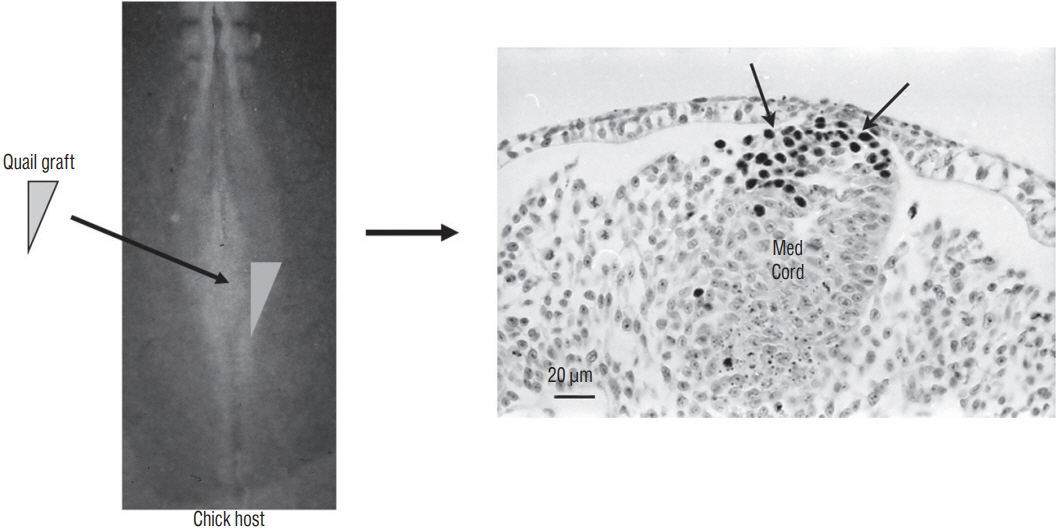
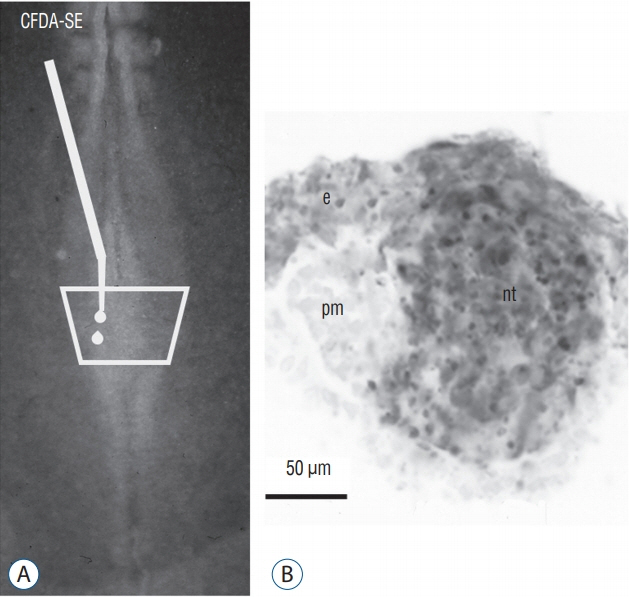
- Secondary neurulation is a morphological process described since the second half of the 19th century; it accounts for the formation of the caudal spinal cord in mammals including humans. A similar process takes place in birds. This form of neurulation is caused by the growth of the tail bud region, the most caudal axial region of the embryo. Experimental work in different animal species leads to questioning dogmas widely disseminated in the medical literature. Thus, it is clearly established that the tail bud is not a mass of undifferentiated pluripotent cells but is made up of a juxtaposition of territories whose fate is different. The lumens of the two tubes generated by the two modes of neurulation are continuous. There seem to be multiple cavities in the human embryo, but discrepancies exist according to the authors. Finally, the tissues that generate the secondary neural tube are initially located in the most superficial layer of the embryo. These cells must undergo internalization to generate the secondary neurectoderm. A defect in internalization could lead to an open neural tube defect that contradicts the dogma that a secondary neurulation defect is closed by definition.
Figure
Reference
-
References
1. Beck CW, Slack JM. Analysis of the developing Xenopus tail bud reveals separate phases of gene expression during determination and outgrowth. Mech Dev. 72:41–52. 1998.
Article2. Bijtel JH. Über die Entwicklung des Schwanzes bei Amphibien. Wilhelm Roux Arch Entwickl Mech Org. 125:448–486. 1931.
Article3. Bouldin CM, Manning AJ, Peng YH, Farr GH 3rd, Hung KL, Dong A, et al. Wnt signaling and tbx16 form a bistable switch to commit bipotential progenitors to mesoderm. Development. 142:2499–2507. 2015.4. Braun M. Entwickelungsvorgänge am Schwanzende bei einigen Säugethieren mit Berücksichtigungder Verhältnisse beim Menschen. Arch f Anat u Phys, Anat Abt. 6:207–241. 1882.5. Cambray N, Wilson V. Axial progenitors with extensive potency are localised to the mouse chordoneural hinge. Development. 129:4855–4866. 2002.
Article6. Catala M, Teillet MA, De Robertis EM, Le Douarin ML. A spinal cord fate map in the avian embryo: while regressing, Hensen’s node lays down the notochord and floor plate thus joining the spinal cord lateral walls. Development. 122:2599–2610. 1996.
Article7. Catala M, Teillet MA, Le Douarin NM. Organization and development of the tail bud analyzed with the quail-chick chimaera system. Mech Dev. 51:51–65. 1995.
Article8. Criley BB. Analysis of the embryonic sources and mechanisms of development of posterior levels of chick neural tubes. J Morphol. 128:465–501. 1969.
Article9. Dady A, Havis E, Escriou V, Catala M, Duband JL. Junctional neurulation: a unique developmental program shaping a discrete region of the spinal cord highly susceptible to neural tube defects. J Neurosci. 34:13208–13221. 2014.
Article10. Davis RL, Kirschner MW. The fate of cells in the tailbud of Xenopus laevis. Development. 127:255–267. 2000.
Article11. Gasser E. Der Primitivstreifen bei Vogelembryonen (Huhn und Gans). Schriften der Gesellschaft zur Beförderung der gesammten Naturwissenschaften zu Marburg. 2(Suppl 1):1–98. 1879.
Article12. Gofflot F, Hall M, Morriss-Kay GM. Genetic patterning of the developing mouse tail at the time of posterior neuropore closure. Dev Dyn. 210:431–445. 1997.
Article13. Gont LK, Steinbeisser H, Blumberg B, de Robertis EM. Tail formation as a continuation of gastrulation: the multiple cell populations of the Xenopus tailbud derive from the late blastopore lip. Development. 119:991–1004. 1993.
Article14. Griffith CM, Wiley MJ. The distribution of cell surface glycoconjugates during mouse secondary neurulation. Anat Embryol (Berl). 180:567–575. 1989.
Article15. Griffith CM, Wiley MJ, Sanders EJ. The vertebrate tail bud: three germ layers from one tissue. Anat Embryol (Berl). 185:101–113. 1992.
Article16. Holmdahl DE. Die zweifache Bildungsweise des zentralen Nervensystem bei den Wirbeltieren. Eine formgeschichtliche und materialgeschichtliche Analyse. Wilhelm Roux Arch Entwickl Mech Org. 129:206–254. 1933.
Article17. Hughes AF, Freeman RB. Comparative remarks on the development of the tail cord among higher vertebrates. J Embryol Exp Morphol. 32:355–363. 1974.
Article18. Kanki JP, Ho RK. The development of the posterior body in zebrafish. Development. 124:881–893. 1997.
Article19. Knezevic V, De Santo R, Mackem S. Continuing organizer function during chick tail development. Development. 125:1791–1801. 1998.
Article20. Kostović-Knezević L, Gajović S, Svajger A. Morphogenetic features in the tail region of the rat embryo. Int J Dev Biol. 35:191–195. 1991.21. Kunitomo K. The development and reduction of the tail and of the caudal end of the spinal cord. Contrib Embryol. 8:161–198. 1918.22. Lemire RJ. Variations in development of the caudal neural tube in human embryos (horizons XIV-XXI). Teratology. 2:361–369. 1969.
Article23. Müller F, O’Rahilly R. The development of the human brain, the closure of the caudal neuropore, and the beginning of secondary neurulation at stage 12. Anat Embryol (Berl). 176:413–430. 1987.
Article24. Müller F, O’Rahilly R. The development of the human brain from a closed neural tube at stage 13. Anat Embryol (Berl). 177:203–224. 1988.
Article25. Nievelstein RA, Hartwig NG, Vermeij-Keers C, Valk J. Embryonic development of the mammalian caudal neural tube. Teratology. 48:21–31. 1993.
Article26. O’Shea KS. Differential deposition of basement membrane components during formation of the caudal neural tube in the mouse embryo. Development. 99:509–519. 1987.
Article27. Pasteels J. Etudes sur la gastrulation des vertébrés méroblastiques. III. Oiseaux. IV Conclusions générales. Arch Biol. 48:381–488. 1937.28. Row RH, Tsotras SR, Goto H, Martin BL. The zebrafish tailbud contains two independent populations of midline progenitor cells that maintain long-term germ layer plasticity and differentiate in response to local signaling cues. Development. 143:244–254. 2016.29. Saitsu H, Yamada S, Uwabe C, Ishibashi M, Shiota K. Development of the posterior neural tube in human embryos. Anat Embryol (Berl). 209:107–117. 2004.
Article30. Schoenwolf GC. Tail (end) bud contributions to the posterior region of the chick embryo. J Exp Zool. 201:227–245. 1977.
Article31. Schoenwolf GC. Effects of complete tail bud extirpation on early development of the posterior region of the chick embryo. Anat Rec. 192:289–295. 1978.
Article32. Schoenwolf GC. Histological and ultrastructural observations of tail bud formation in the chick embryo. Anat Rec. 193:131–147. 1979.
Article33. Schoenwolf GC. Histological and ultrastructural studies of secondary neurulation in mouse embryos. Am J Anat. 169:361–376. 1984.
Article34. Schoenwolf GC, Chandler NB, Smith JL. Analysis of the origins and early fates of neural crest cells in caudal regions of avian embryos. Dev Biol. 110:467–479. 1985.
Article35. Schoenwolf GC, Delongo J. Ultrastructure of secondary neurulation in the chick embryo. Am J Anat. 158:43–63. 1980.
Article36. Schoenwolf GC, Nichols DH. Histological and ultrastructural studies on the origin of caudal neural crest cells in mouse embryos. J Comp Neurol. 222:496–505. 1984.
Article37. Schumacher S. Über die sogenannte Vervielfachung des Medullarrohres (bzw. des Canalis centralis) bei Embryonen. Z Mikrosk Anat Forsch. 10:83–109. 1927.38. Shedden PM, Wiley MJ. Early stages of development in the caudal neural tube of the Golden Syrian hamster (Mesocricetus auratus). Anat Rec. 219:180–185. 1987.
Article39. Shih J, Fraser SE. Characterizing the zebrafish organizer: microsurgical analysis at the early-shield stage. Development. 122:1313–1322. 1996.
Article40. Shimokita E, Takahashi Y. Secondary neurulation: fate-mapping and gene manipulation of the neural tube in tail bud. Dev Growth Differ. 53:401–410. 2011.
Article41. Taniguchi Y, Kurth T, Weiche S, Reichelt S, Tazaki A, Perike S, et al. The posterior neural plate in axolotl gives rise to neural tube or turns anteriorly to form somites of the tail and posterior trunk. Dev Biol. 422:155–170. 2017.
Article42. Tucker AS, Slack JM. The Xenopus laevis tail-forming region. Development. 121:249–262. 1995.
Article43. Wilson V, Beddington RS. Cell fate and morphogenetic movement in the late mouse primitive streak. Mech Dev. 55:79–89. 1996.
Article44. Wilson V, Olivera-Martinez I, Storey KG. Stem cells, signals and vertebrate body axis extension. Development. 136:1591–1604. 2009.
Article45. Yang HJ, Lee DH, Lee YJ, Chi JG, Lee JY, Phi JH, et al. Secondary neurulation of human embryos: morphological changes and the expression of neuronal antigens. Childs Nerv Syst. 30:73–82. 2014.
Article46. Zwilling E. Restitution of the tail in the early chick embryo. J Exp Zool. 91:453–463. 1942.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Perspectives : The Role of Clinicians in Understanding Secondary Neurulation
- Junctional Neurulation : A Junction between Primary and Secondary Neural Tubes
- Cell Lineage, Self-Renewal, and Epithelial-to-Mesenchymal Transition during Secondary Neurulation
- Disorders of Secondary Neurulation : Mainly Focused on Pathoembryogenesis
- Junctional Neural Tube Defect