J Korean Neurosurg Soc.
2020 May;63(3):321-326. 10.3340/jkns.2020.0063.
Terminal Myelocystocele : Pathoembryogenesis and Clinical Features
- Affiliations
-
- 1Division of Pediatric Neurosurgery, Seoul National University Children's Hospital, Seoul, Korea
- 2Department of Anatomy and Cell Biology, Seoul National University College of Medicine, Seoul, Korea
- KMID: 2501718
- DOI: http://doi.org/10.3340/jkns.2020.0063
Abstract
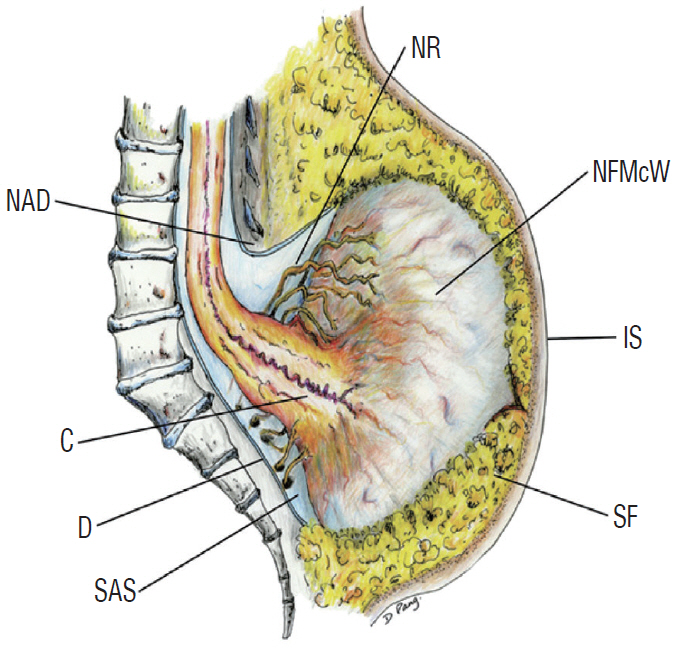
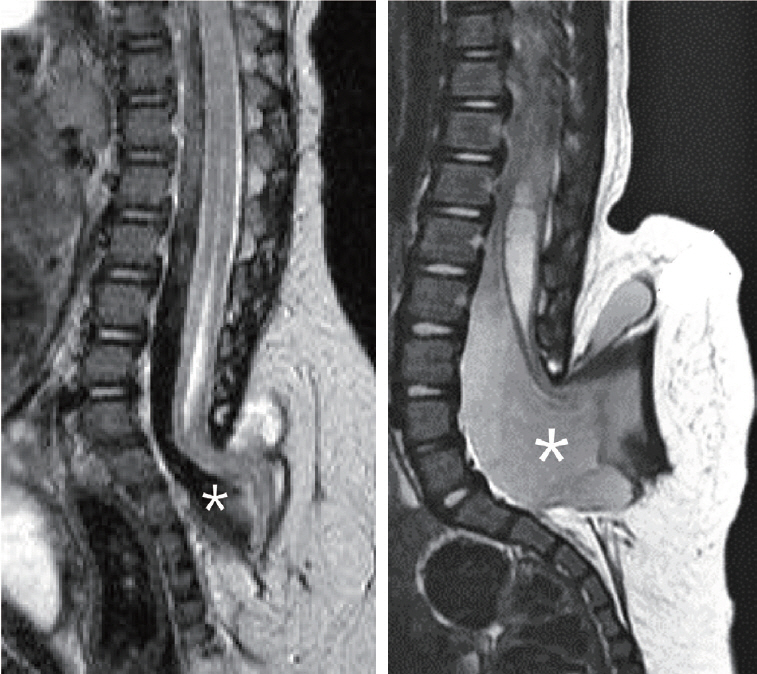
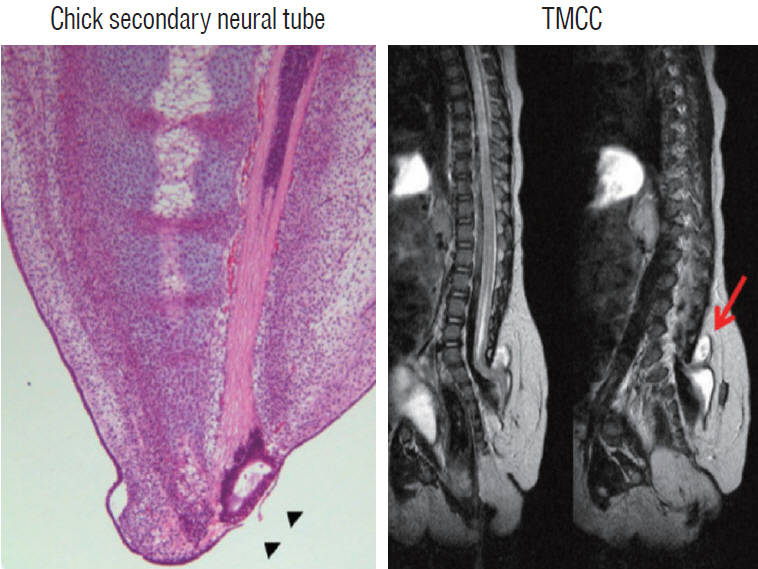
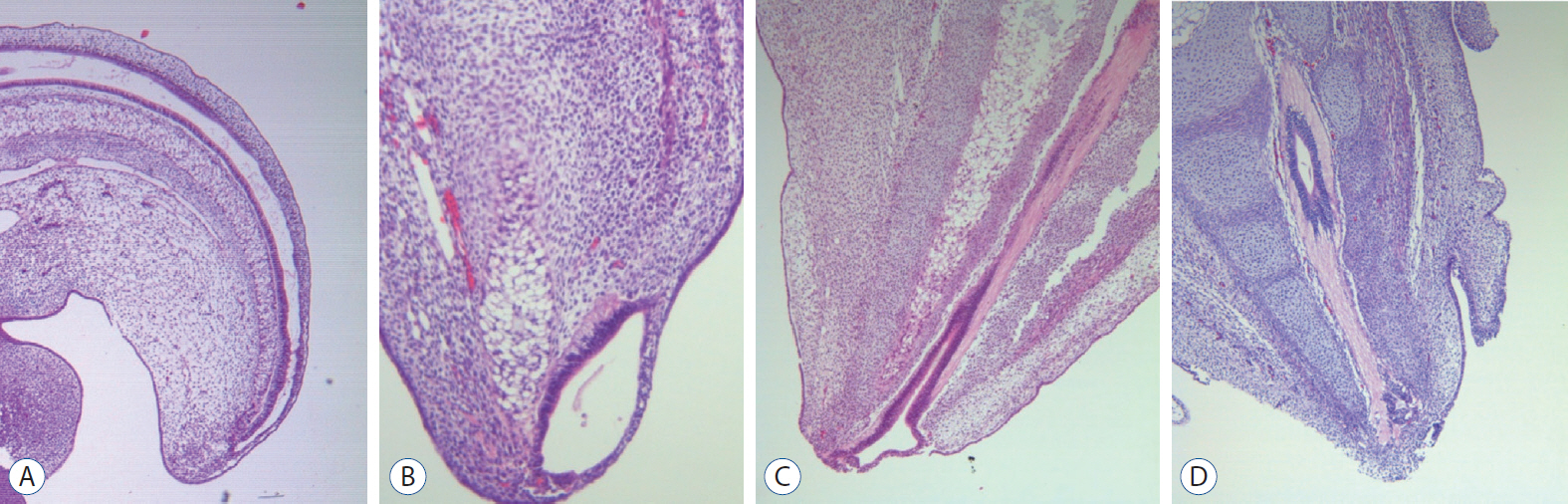
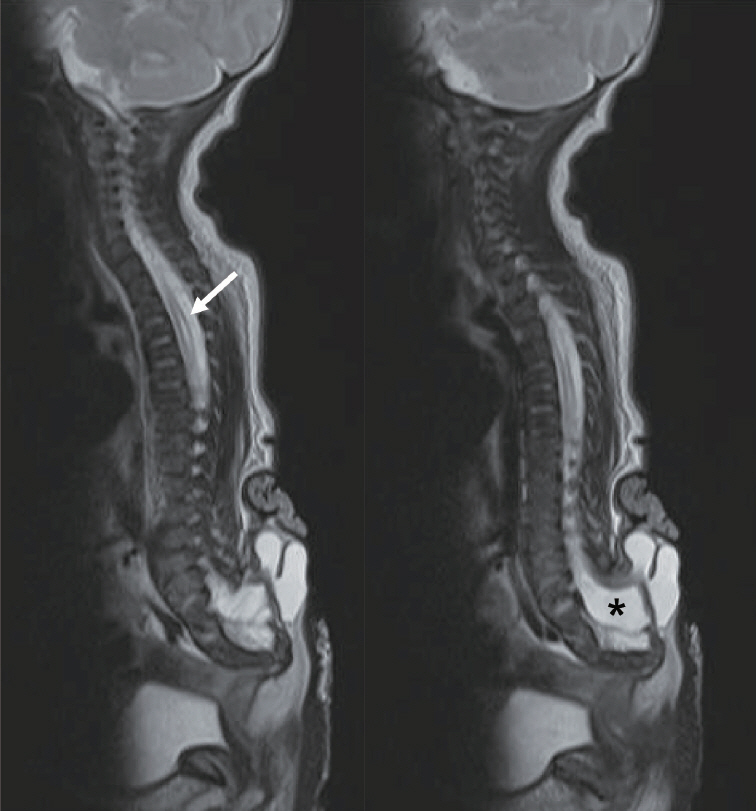
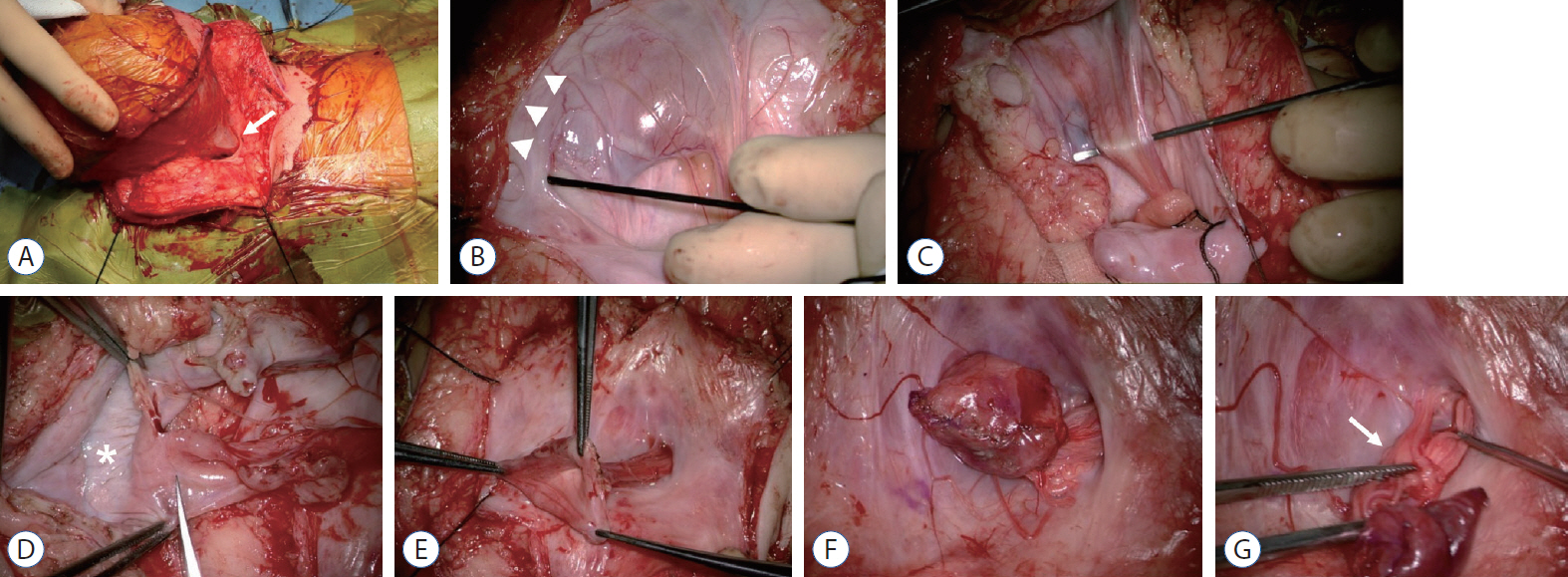
- There has been confusion in the classification of terminal myelocystocele (TMCC) due to its diverse morphology and vague pathoembryogenesis. TMCC could be summarized as having the essential features of an elongated caudal spinal cord extruding out of the dorsal extraspinal space that fuses with the subcutaneous fat, which is in the shape of a trumpet-shaped cerebrospinal fluid-filled cyst. The extraspinal portion of the extruded spinal cord is nonfunctional. The morphological features suggest that TMCC is formed during secondary neurulation, specifically the failure of the degeneration of the secondary neural tube near the time of the terminal balloon. This review discusses the definition, as well as the clinical and surgical features, of TMCC with special emphasis on its pathoembryogenesis.
Keyword
Figure
Reference
-
References
1. Barkovich AJ, Kuzniecky RI, Dobyns WB. Radiologic classification of malformations of cortical development. Curr Opin Neurol. 14:145–149. 2001.
Article2. Byrd SE, Harvey C, McLone DG, Darling CF. Imaging of terminal myelocystoceles. J Natl Med Assoc. 88:510–516. 1996.3. Choi S, McComb JG. Long-term outcome of terminal myelocystocele patients. Pediatr Neurosurg. 32:86–91. 2000.
Article4. Lee JY, Phi JH, Kim SK, Cho BK, Wang KC. Urgent surgery is needed when cyst enlarges in terminal myelocystoceles. Childs Nerv Syst. 27:2149–2153. 2011.
Article5. McLone DG, Naidich TP. Terminal myelocystocele. Neurosurgery. 16:36–43. 1985.
Article6. Pang D, Zovickian J, Lee JY, Moes GS, Wang KC. Terminal myelocystocele: surgical observations and theory of embryogenesis. Neurosurgery. 70:1383–1404. discussion 1404-1405. 2012.7. Tandon V, Garg K, Mahapatra AK. Terminal myelocystocele: a series of 30 cases and review of the literature. Pediatr Neurosurg. 48:229–235. 2012.
Article8. Yang HJ, Lee DH, Lee YJ, Chi JG, Lee JY, Phi JH, et al. Secondary neurulation of human embryos: morphological changes and the expression of neuronal antigens. Childs Nerv Syst. 30:73–82. 2014.
Article9. Yang HJ, Wang KC, Chi JG, Lee MS, Lee YJ, Kim SK, et al. Neural differentiation of caudal cell mass (secondary neurulation) in chick embryos: Hamburger and Hamilton Stages 16-45. Brain Res Dev Brain Res. 142:31–36. 2003.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Terminal myelocystocele: a case report
- Terminal Myelocystocele: A Skin-Covered Lumbosacral Cystic Mass
- Disorders of Secondary Neurulation : Mainly Focused on Pathoembryogenesis
- Enlargement of Extraspinal Cysts in Spinal Dysraphism : A Reason for Early Untethering
- The Impact of Clinical Nurses' Terminal Care Attitude and Spiritual Health on Their Terminal Care Stress