Yeungnam Univ J Med.
2021 Apr;38(2):160-164. 10.12701/yujm.2020.00339.
A new type of oculocutaneous albinism with a novel OCA2 mutation
- Affiliations
-
- 1Department of Pediatrics, Kyungpook National University Hospital, Daegu, Korea
- 2Department of Pediatrics, Daegu Fatima Hospital, Daegu, Korea
- 3Departments of Pediatrics, Yeungnam University College of Medicine, Daegu, Korea
- 4Department of Pediatrics, School of Medicine, Kyungpook National University, Daegu, Korea
- KMID: 2515192
- DOI: http://doi.org/10.12701/yujm.2020.00339
Abstract
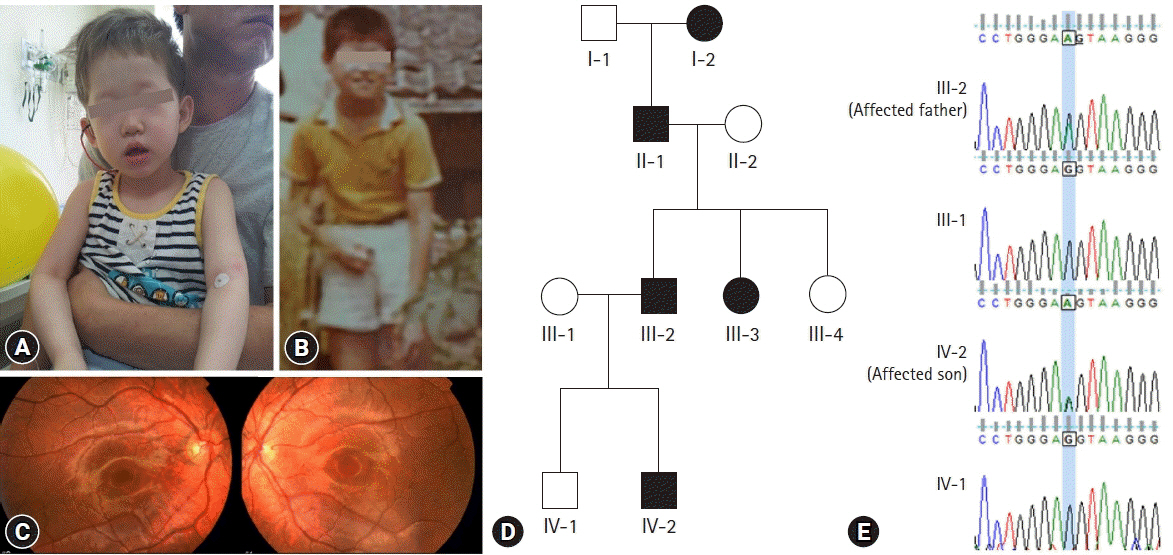
- Oculocutaneous albinism (OCA) is a group of rare genetically heterogeneous disorders, characterized by hypopigmentation of the eyes, skin, and hair, which result in ocular abnormalities and a risk of developing skin cancer. Currently, there is no ophthalmologic procedure or drug that prevents the clinical features of OCA. Here, we report a new type of OCA in two, unrelated Korean families with the same OCA2 mutation. Affected individuals in this study are different from those of previous reports in two aspects: an inheritance pattern and clinical presentation. All reported patients with OCA have shown an autosomal recessive inheritance pattern, while our patients showed an autosomal dominant inheritance pattern. Small amounts of pigment can be acquired with age in OCA, but there is no substantial variation from adolescence to adulthood in this regard. A case where the patient attained normal pigmentation levels has never been reported. However, our patients displayed completely normal pigmentation in their late twenties. Whole exome sequencing and in-silico analysis revealed a novel mutation, OCA2 c.2338G>A p.(G780S) (NM_000275) with a high likelihood of pathogenicity. Sanger sequencing of p.G780S identified the same mutation in the affected individuals, which was not found in the family members with normal phenotype. We hypothesize that OCA2 G780S not only acts as a pathogenic variant of OCA but also induces pigmentation by enhancing the melanogenesis gene expression of other modifier genes, such as SLC45A2 and TPC2. These findings may provide further understanding of melanin biosynthesis and new treatment methods for OCA.
Figure
Reference
-
References
1. Johanson HC, Chen W, Wicking C, Sturm RA. Inheritance of a novel mutated allele of the OCA2 gene associated with high incidence of oculocutaneous albinism in a Polynesian community. J Hum Genet. 2010; 55:103–11.
Article2. Prota G. The role of peroxidase in melanogenesis revisited. Pigment Cell Res. 1992; Suppl 2:25–31.
Article3. Wang H, Wan Y, Yang Y, Li H, Mao L, Gao S, et al. Novel compound heterozygous mutations in OCA2 gene associated with non-syndromic oculocutaneous albinism in a Chinese Han patient: a case report. BMC Med Genet. 2019; 20:130.
Article4. Dessinioti C, Stratigos AJ, Rigopoulos D, Katsambas AD. A review of genetic disorders of hypopigmentation: lessons learned from the biology of melanocytes. Exp Dermatol. 2009; 18:741–9.
Article5. Yang Q, Yi S, Li M, Xie B, Luo J, Wang J, et al. Genetic analyses of oculocutaneous albinism types 1 and 2 with four novel mutations. BMC Med Genet. 2019; 20:106.
Article6. Inagaki K, Suzuki T, Shimizu H, Ishii N, Umezawa Y, Tada J, et al. Oculocutaneous albinism type 4 is one of the most common types of albinism in Japan. Am J Hum Genet. 2004; 74:466–71.
Article7. Gardner JM, Nakatsu Y, Gondo Y, Lee S, Lyon MF, King RA, et al. The mouse pink-eyed dilution gene: association with human Prader-Willi and Angelman syndromes. Science. 1992; 257:1121–4.
Article8. Ramsay M, Colman MA, Stevens G, Zwane E, Kromberg J, Farrall M, et al. The tyrosinase-positive oculocutaneous albinism locus maps to chromosome 15q11.2-q12. Am J Hum Genet. 1992; 51:879–84.9. Rinchik EM, Bultman SJ, Horsthemke B, Lee ST, Strunk KM, Spritz RA, et al. A gene for the mouse pink-eyed dilution locus and for human type II oculocutaneous albinism. Nature. 1993; 361:72–6.
Article10. Stevens G, Ramsay M, Jenkins T. Oculocutaneous albinism (OCA2) in sub-Saharan Africa: distribution of the common 2.7-kb P gene deletion mutation. Hum Genet. 1997; 99:523–7.
Article11. Stevens G, van Beukering J, Jenkins T, Ramsay M. An intragenic deletion of the P gene is the common mutation causing tyrosinase-positive oculocutaneous albinism in southern African Negroids. Am J Hum Gene. 1995; 56:586–91.12. Ancans J, Tobin DJ, Hoogduijn MJ, Smit NP, Wakamatsu K, Thody AJ. Melanosomal pH controls rate of melanogenesis, eumelanin/phaeomelanin ratio and melanosome maturation in melanocytes and melanoma cells. Exp Cell Res. 2001; 268:26–35.
Article13. Ancans J, Hoogduijn MJ, Thody AJ. Melanosomal pH, pink locus protein and their roles in melanogenesis. J Invest Dermatol. 2001; 117:158–9.
Article14. Wiriyasermkul P, Moriyama S, Nagamori S. Membrane transport proteins in melanosomes: regulation of ions for pigmentation. Biochim Biophys Acta Biomembr. 2020; 1862:183318.
Article15. Lee ST, Nicholls RD, Bundey S, Laxova R, Musarella M, Spritz RA. Mutations of the P gene in oculocutaneous albinism, ocular albinism, and Prader-Willi syndrome plus albinism. N Engl J Med. 1994; 330:529–34.
Article16. Saitoh S, Oiso N, Wada T, Narazaki O, Fukai K. Oculocutaneous albinism type 2 with a P gene missense mutation in a patient with Angelman syndrome. J Med Genet. 2000; 37:392–4.
Article17. Suzuki T, Miyamura Y, Matsunaga J, Shimizu H, Kawachi Y, Ohyama N, et al. Six novel P gene mutations and oculocutaneous albinism type 2 frequency in Japanese albino patients. J Invest Dermatol. 2003; 120:781–3.
Article18. Wei AH, Yang XM, Lian S, Li W. Genetic analyses of Chinese patients with digenic oculocutaneous albinism. Chin Med J. 2013; 126:226–30.19. Wang Y, Wang Z, Chen M, Fan N, Yang J, Liu L, et al. Mutational analysis of the TYR and OCA2 genes in four chinese families with oculocutaneous albinism. PLoS One. 2015; 10:e0125651.
Article20. Lewis RA. Oculocutaneous albinism type 2. In : Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, editors. GeneReviews®. Seattle (WA): University of Washington;1993.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Oculocutaneous Albinism Type 1 Diagnosed by Genetic Study in a Newborn Infant
- Reevaluation of Single Nucleotide Polymorphism of OCA2 in Koreans
- A Case of Oculocutaneous Albinism
- A Case of Oculocutaneous Albinism 1A Accompanying with Tyrosinase Mutation
- Two Cases of Oculocutaneous Albinism with Congenital Nystagmus