Korean J Physiol Pharmacol.
2021 May;25(3):189-195. 10.4196/kjpp.2021.25.3.189.
Fluoxetine affects cytosolic cAMP, ATP, Ca2+ responses to forskolin, and survival of human ovarian granulosa tumor COV434 cells
- Affiliations
-
- 1Physiologie de la Reproduction & des Comportements Laboratory, Centre National de la Recherche Scientifique (CNRS), Institut National de la Recherche Agronomique & Environnementale (INRAe), University of Tours, Nouzilly 37380, France
- 2Faculty of Natural Sciences, Quy Nhon University, Quy Nhon 820000, Vietnam
- KMID: 2514944
- DOI: http://doi.org/10.4196/kjpp.2021.25.3.189
Abstract
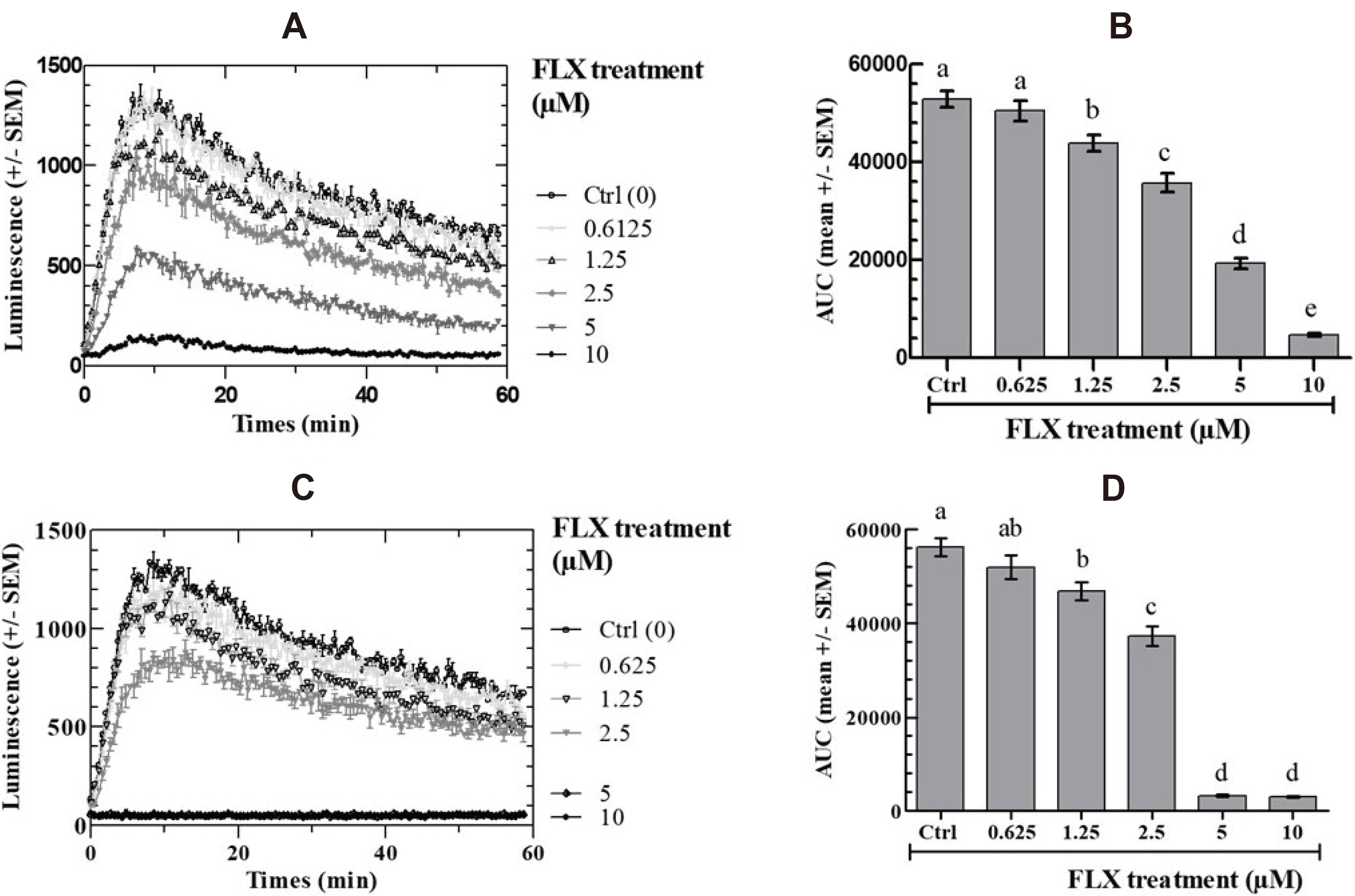
- Fluoxetine (FLX), a selective serotonin reuptake inhibitor antidepressant, exhibits various other mechanisms of action in numerous cell types and has been shown to induce cell death in cancer cells, paving the way for its potential use in cancer therapy. The aim of this study was to determine the off-target effects of the anti-depressant drug FLX, on the human ovarian granulosa tumor COV434 cells stimulated by forskolin (FSK), by measuring the real-time kinetics of intracellular cyclic AMP (cAMP), ATP level, cytoplasmic calcium ([Ca 2+ ] cyt ) and survival of COV434 cells. We show that incubating COV434 cells with FLX (between 0.6 and 10 ㎛) induces a decrease in intracellular cAMP response to FSK, a drop in ATP content and stimulates cytoplasmic Ca 2+ accumulation in COV434 cells. Only the highest concentrations of FLX (5–10 ㎛) diminished cell viability. The present report is the first to identify an action mechanism of FLX in human tumor ovarian cells COV434 cells and thus opening the way to potential use of fluoxetine as a complementary tool, in granulosa tumor treatments.
Keyword
Figure
Reference
-
1. Wong DT, Bymaster FP, Engleman EA. 1995; Prozac (fluoxetine, Lilly 110140), the first selective serotonin uptake inhibitor and an antidepressant drug: twenty years since its first publication. Life Sci. 57:411–441. DOI: 10.1016/0024-3205(95)00209-O. PMID: 7623609.
Article2. Stark P, Fuller RW, Wong DT. 1985; The pharmacologic profile of fluoxetine. J Clin Psychiatry. 46(3 Pt 2):7–13. PMID: 3871767.3. Fuller RW, Wong DT, Robertson DW. 1991; Fluoxetine, a selective inhibitor of serotonin uptake. Med Res Rev. 11:17–34. DOI: 10.1002/med.2610110103. PMID: 1994152.
Article4. Pancrazio JJ, Kamatchi GL, Roscoe AK, Lynch C 3rd. 1998; Inhibition of neuronal Na+ channels by antidepressant drugs. J Pharmacol Exp Ther. 284:208–214. PMID: 9435180.5. Deák F, Lasztóczi B, Pacher P, Petheö GL, Kecskeméti V, Spät A. 2000; Inhibition of voltage-gated calcium channels by fluoxetine in rat hippocampal pyramidal cells. Neuropharmacology. 39:1029–1036. DOI: 10.1016/S0028-3908(99)00206-3. PMID: 10727713.
Article6. Mukherjee J, Das MK, Yang ZY, Lew R. 1998; Evaluation of the binding of the radiolabeled antidepressant drug, 18F-fluoxetine in the rodent brain: an in vitro and in vivo study. Nucl Med Biol. 25:605–610. DOI: 10.1016/S0969-8051(98)00043-2. PMID: 9804041.
Article7. Francis SH, Corbin JD. 1994; Structure and function of cyclic nucleotide-dependent protein kinases. Annu Rev Physiol. 56:237–272. DOI: 10.1146/annurev.ph.56.030194.001321. PMID: 8010741.
Article8. Maurice DH, Palmer D, Tilley DG, Dunkerley HA, Netherton SJ, Raymond DR, Elbatarny HS, Jimmo SL. 2003; Cyclic nucleotide phosphodiesterase activity, expression, and targeting in cells of the cardiovascular system. Mol Pharmacol. 64:533–546. DOI: 10.1124/mol.64.3.533. PMID: 12920188.
Article9. Jin L, Hill KK, Filak H, Mogan J, Knowles H, Zhang B, Perraud AL, Cambier JC, Lenz LL. 2011; MPYS is required for IFN response factor 3 activation and type I IFN production in the response of cultured phagocytes to bacterial second messengers cyclic-di-AMP and cyclic-di-GMP. J Immunol. 187:2595–2601. DOI: 10.4049/jimmunol.1100088. PMID: 21813776. PMCID: PMC3159690.
Article10. Berridge MJ, Bootman MD, Roderick HL. 2003; Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 4:517–529. DOI: 10.1038/nrm1155. PMID: 12838335.
Article11. Berridge MJ, Lipp P, Bootman MD. 2000; The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 1:11–21. DOI: 10.1038/35036035. PMID: 11413485.
Article12. Carafoli E, Santella L, Branca D, Brini M. 2001; Generation, control, and processing of cellular calcium signals. Crit Rev Biochem Mol Biol. 36:107–260. DOI: 10.1080/20014091074183. PMID: 11370791.
Article13. Duchen MR. 1999; Contributions of mitochondria to animal physiology: from homeostatic sensor to calcium signalling and cell death. J Physiol. 516(Pt 1):1–17. DOI: 10.1111/j.1469-7793.1999.001aa.x. PMID: 10066918. PMCID: PMC2269224.
Article14. Duchen MR. 2000; Mitochondria and calcium: from cell signalling to cell death. J Physiol. 529(Pt 1):57–68. DOI: 10.1111/j.1469-7793.2000.00057.x. PMID: 11080251. PMCID: PMC2270168.
Article15. Nguyen TMD, Klett D, Filliatreau L, Combarnous Y. 2019; Inhibition by fluoxetine of LH-stimulated cyclic AMP synthesis in tumor Leydig cells partly involves AMPK activation. PLoS One. 14:e0217519. DOI: 10.1371/journal.pone.0217519. PMID: 31163038. PMCID: PMC6548379.
Article16. Zhang H, Vollmer M, De Geyter M, Litzistorf Y, Ladewig A, Dürrenberger M, Guggenheim R, Miny P, Holzgreve W, De Geyter C. 2000; Characterization of an immortalized human granulosa cell line (COV434). Mol Hum Reprod. 6:146–153. DOI: 10.1093/molehr/6.2.146. PMID: 10655456.
Article17. Curti C, Mingatto FE, Polizello AC, Galastri LO, Uyemura SA, Santos AC. 1999; Fluoxetine interacts with the lipid bilayer of the inner membrane in isolated rat brain mitochondria, inhibiting electron transport and F1F0-ATPase activity. Mol Cell Biochem. 199:103–109. DOI: 10.1023/A:1006912010550. PMID: 10544958.18. Gincel D, Zaid H, Shoshan-Barmatz V. 2001; Calcium binding and translocation by the voltage-dependent anion channel: a possible regulatory mechanism in mitochondrial function. Biochem J. 358(Pt 1):147–155. DOI: 10.1042/bj3580147. PMCID: PMC1222042. PMID: 11485562.
Article19. Rostovtseva T, Colombini M. 1997; VDAC channels mediate and gate the flow of ATP: implications for the regulation of mitochondrial function. Biophys J. 72:1954–1962. DOI: 10.1016/S0006-3495(97)78841-6. PMID: 9129800. PMCID: PMC1184392.
Article20. Hodge T, Colombini M. 1997; Regulation of metabolite flux through voltage-gating of VDAC channels. J Membr Biol. 157:271–279. DOI: 10.1007/s002329900235. PMID: 9178614.
Article21. Shoshan-Barmatz V, Gincel D. 2003; The voltage-dependent anion channel: characterization, modulation, and role in mitochondrial function in cell life and death. Cell Biochem Biophys. 39:279–292. DOI: 10.1385/CBB:39:3:279. PMID: 14716081.
Article22. Nahon E, Israelson A, Abu-Hamad S, Varda SB. 2005; Fluoxetine (Prozac) interaction with the mitochondrial voltage-dependent anion channel and protection against apoptotic cell death. FEBS Lett. 579:5105–5110. DOI: 10.1016/j.febslet.2005.08.020. PMID: 16139271.
Article23. Charles E, Hammadi M, Kischel P, Delcroix V, Demaurex N, Castelbou C, Vacher AM, Devin A, Ducret T, Nunes P, Vacher P. 2017; The antidepressant fluoxetine induces necrosis by energy depletion and mitochondrial calcium overload. Oncotarget. 8:3181–3196. DOI: 10.18632/oncotarget.13689. PMID: 27911858. PMCID: PMC5356874.
Article24. Costa-Junior HM, Mendes AN, Davis GH, da Cruz CM, Ventura AL, Serezani CH, Faccioli LH, Nomizo A, Freire-de-Lima CG, Bisaggio Rda C, Persechini PM. 2009; ATP-induced apoptosis involves a Ca2+-independent phospholipase A2 and 5-lipoxygenase in macrophages. Prostaglandins Other Lipid Mediat. 88:51–61. DOI: 10.1016/j.prostaglandins.2008.09.004. PMID: 18984060.25. Sakanashi Y, Oyama TM, Matsuo Y, Oyama TB, Nishimura Y, Ishida S, Imai S, Okano Y, Oyama Y. 2009; Zn2+, derived from cell preparation, partly attenuates Ca2+-dependent cell death induced by A23187, calcium ionophore, in rat thymocytes. Toxicol In Vitro. 23:338–345. DOI: 10.1016/j.tiv.2008.12.006. PMID: 19124067.26. Kannen V, Hintzsche H, Zanette DL, Silva WA Jr, Garcia SB, Waaga-Gasser AM, Stopper H. 2012; Antiproliferative effects of fluoxetine on colon cancer cells and in a colonic carcinogen mouse model. PLoS One. 7:e50043. DOI: 10.1371/journal.pone.0050043. PMID: 23209640. PMCID: PMC3507893.
Article27. Cloonan SM, Williams DC. 2011; The antidepressants maprotiline and fluoxetine induce Type II autophagic cell death in drug-resistant Burkitt's lymphoma. Int J Cancer. 128:1712–1723. DOI: 10.1002/ijc.25477. PMID: 20503272.
Article28. Lee CS, Kim YJ, Jang ER, Kim W, Myung SC. 2010; Fluoxetine induces apoptosis in ovarian carcinoma cell line OVCAR-3 through reactive oxygen species-dependent activation of nuclear factor-kappaB. Basic Clin Pharmacol Toxicol. 106:446–453. DOI: 10.1111/j.1742-7843.2009.00509.x. PMID: 20050848.29. Brambilla P, Cipriani A, Hotopf M, Barbui C. 2005; Side-effect profile of fluoxetine in comparison with other SSRIs, tricyclic and newer antidepressants: a meta-analysis of clinical trial data. Pharmacopsychiatry. 38:69–77. DOI: 10.1055/s-2005-837806. PMID: 15744630.
Article30. Tarasov AI, Griffiths EJ, Rutter GA. 2012; Regulation of ATP production by mitochondrial Ca2+. Cell Calcium. 52:28–35. DOI: 10.1016/j.ceca.2012.03.003. PMID: 22502861. PMCID: PMC3396849.31. Rizzuto R, Marchi S, Bonora M, Aguiari P, Bononi A, De Stefani D, Giorgi C, Leo S, Rimessi A, Siviero R, Zecchini E, Pinton P. 2009; Ca2+ transfer from the ER to mitochondria: when, how and why. Biochim Biophys Acta. 1787:1342–1351. DOI: 10.1016/j.bbabio.2009.03.015. PMID: 19341702. PMCID: PMC2730423.32. Spät A, Szanda G, Csordás G, Hajnóczky G. 2008; High- and low-calcium-dependent mechanisms of mitochondrial calcium signalling. Cell Calcium. 44:51–63. DOI: 10.1016/j.ceca.2007.11.015. PMID: 18242694. PMCID: PMC2662195.
Article33. Rasola A, Bernardi P. 2011; Mitochondrial permeability transition in Ca2+-dependent apoptosis and necrosis. Cell Calcium. 50:222–233. DOI: 10.1016/j.ceca.2011.04.007. PMID: 21601280.34. Rasola A, Sciacovelli M, Pantic B, Bernardi P. 2010; Signal transduction to the permeability transition pore. FEBS Lett. 584:1989–1996. DOI: 10.1016/j.febslet.2010.02.022. PMID: 20153328. PMCID: PMC2866765.
Article35. Leist M, Single B, Castoldi AF, Kühnle S, Nicotera P. 1997; Intracellular adenosine triphosphate (ATP) concentration: a switch in the decision between apoptosis and necrosis. J Exp Med. 185:1481–1486. DOI: 10.1084/jem.185.8.1481. PMID: 9126928. PMCID: PMC2196283.
Article36. Bernardi P, Rasola A. 2007; Calcium and cell death: the mitochondrial connection. Subcell Biochem. 45:481–506. DOI: 10.1007/978-1-4020-6191-2_18. PMID: 18193649.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Juvenile Cystic Granulosa Cell Tumor of the Ovary
- Efffects of Fluoxetine on ATP-induced Calcium Signaling in PC12 Cells
- A Case of Granulosa Cell Tumor of Ovary with Distant Metastasis
- P2 Receptor-mediated Inhibition of Vasopressin-stimulated Fluid Transport and cAMP Responses in AQP2-transfected MDCK Cells
- A Case of Simultaneous Occurrence of Granulosa Cell Tumor and Mature Cystic Teratoma in Same Ovary