Korean J Physiol Pharmacol.
2009 Feb;13(1):9-14. 10.4196/kjpp.2009.13.1.9.
P2 Receptor-mediated Inhibition of Vasopressin-stimulated Fluid Transport and cAMP Responses in AQP2-transfected MDCK Cells
- Affiliations
-
- 1Department of Physiology, Pusan National University School of Medicine, Busan 609-739, Korea. swoo@pusan.ac.kr
- 2Department of Physiology, Dong-A University College of Medicine, Busan 602-714, Korea.
- KMID: 2285365
- DOI: http://doi.org/10.4196/kjpp.2009.13.1.9
Abstract
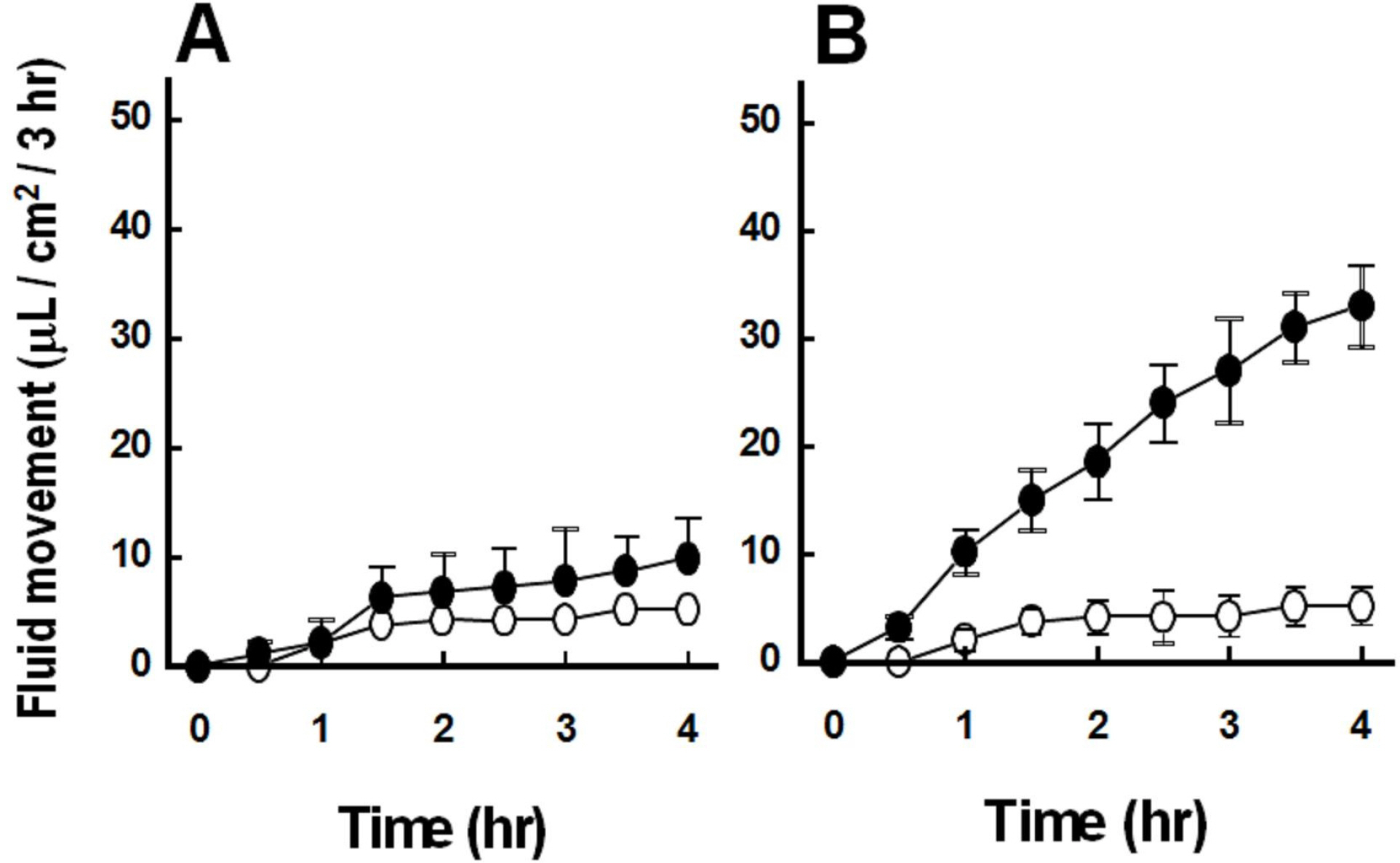
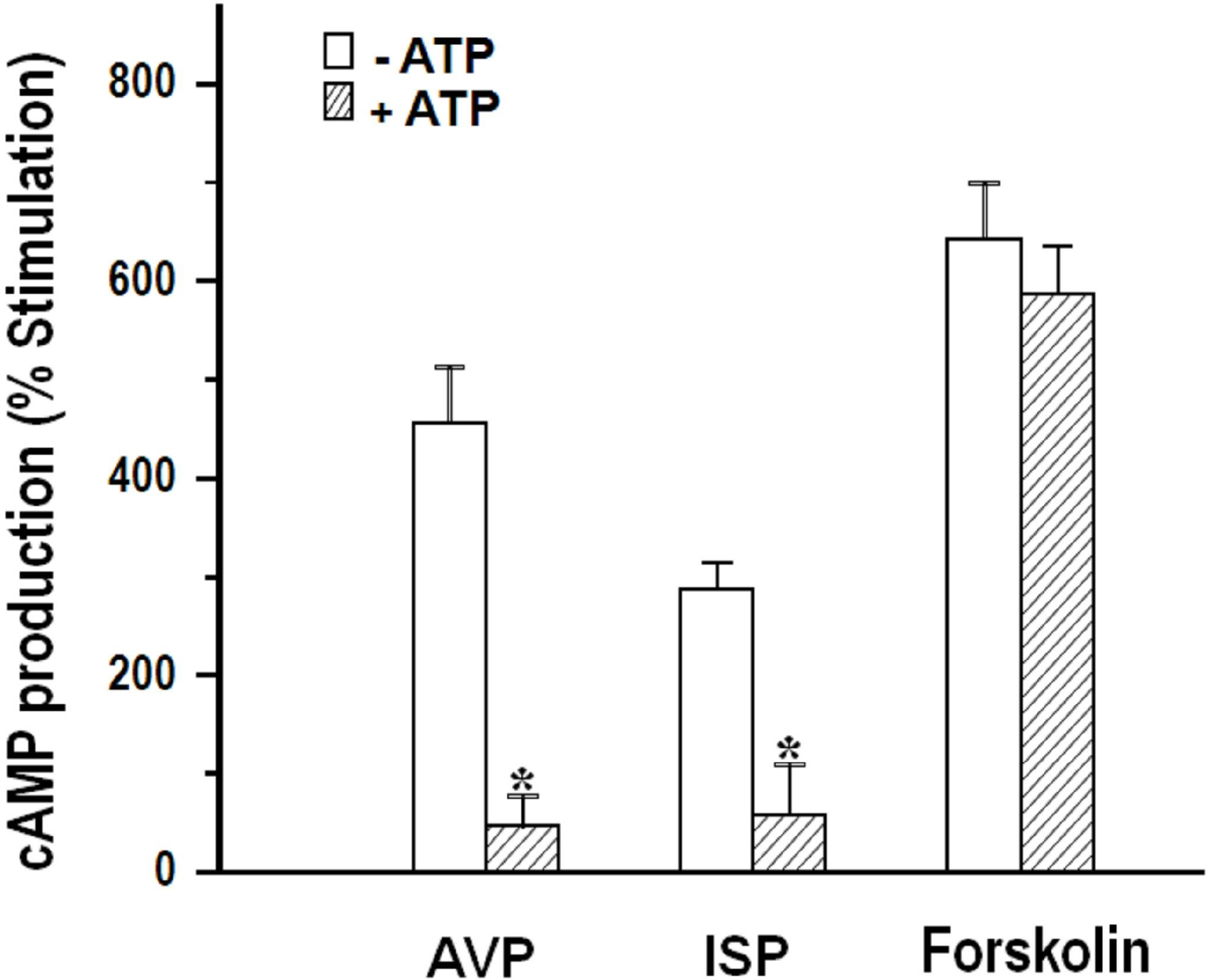
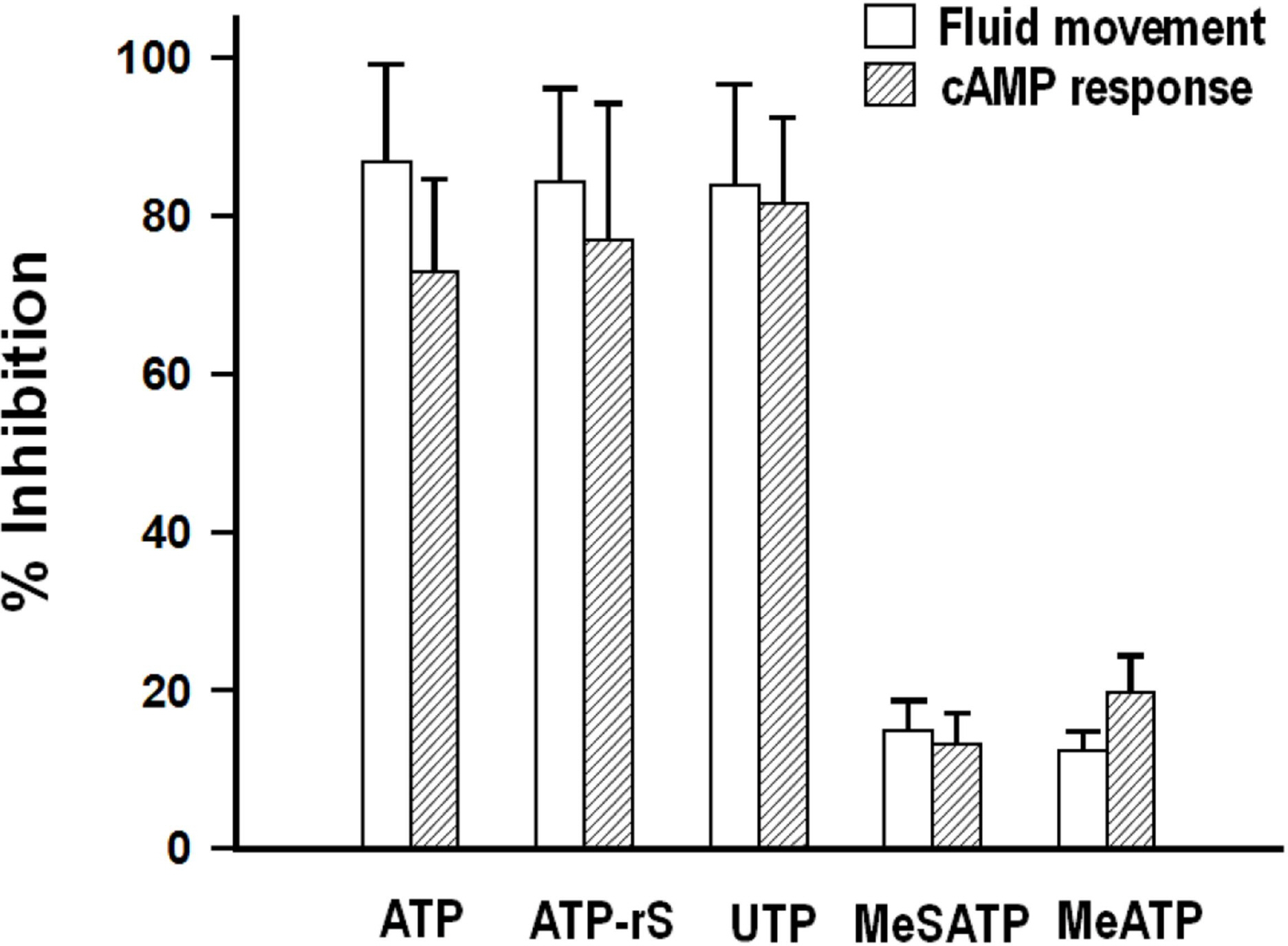
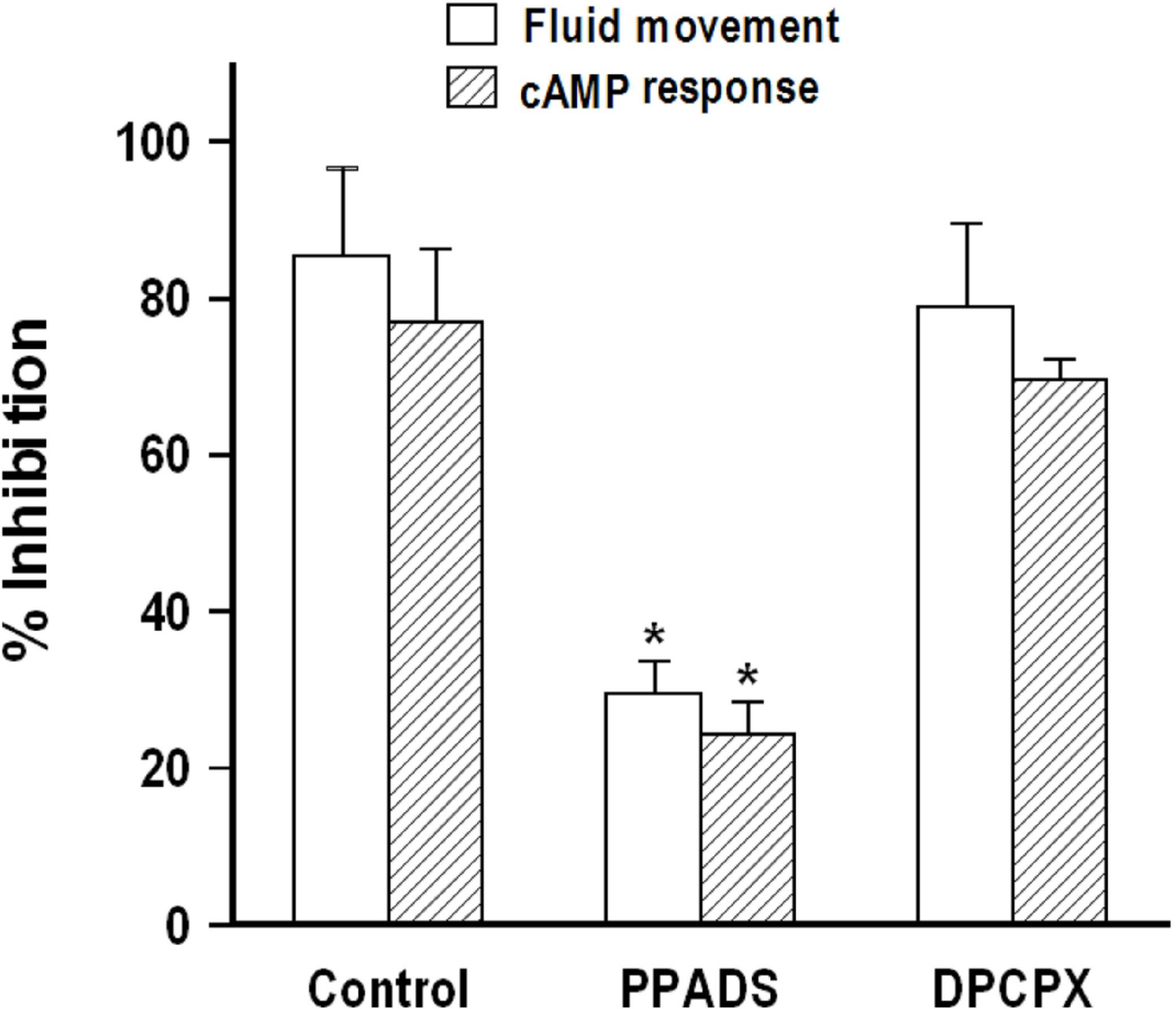
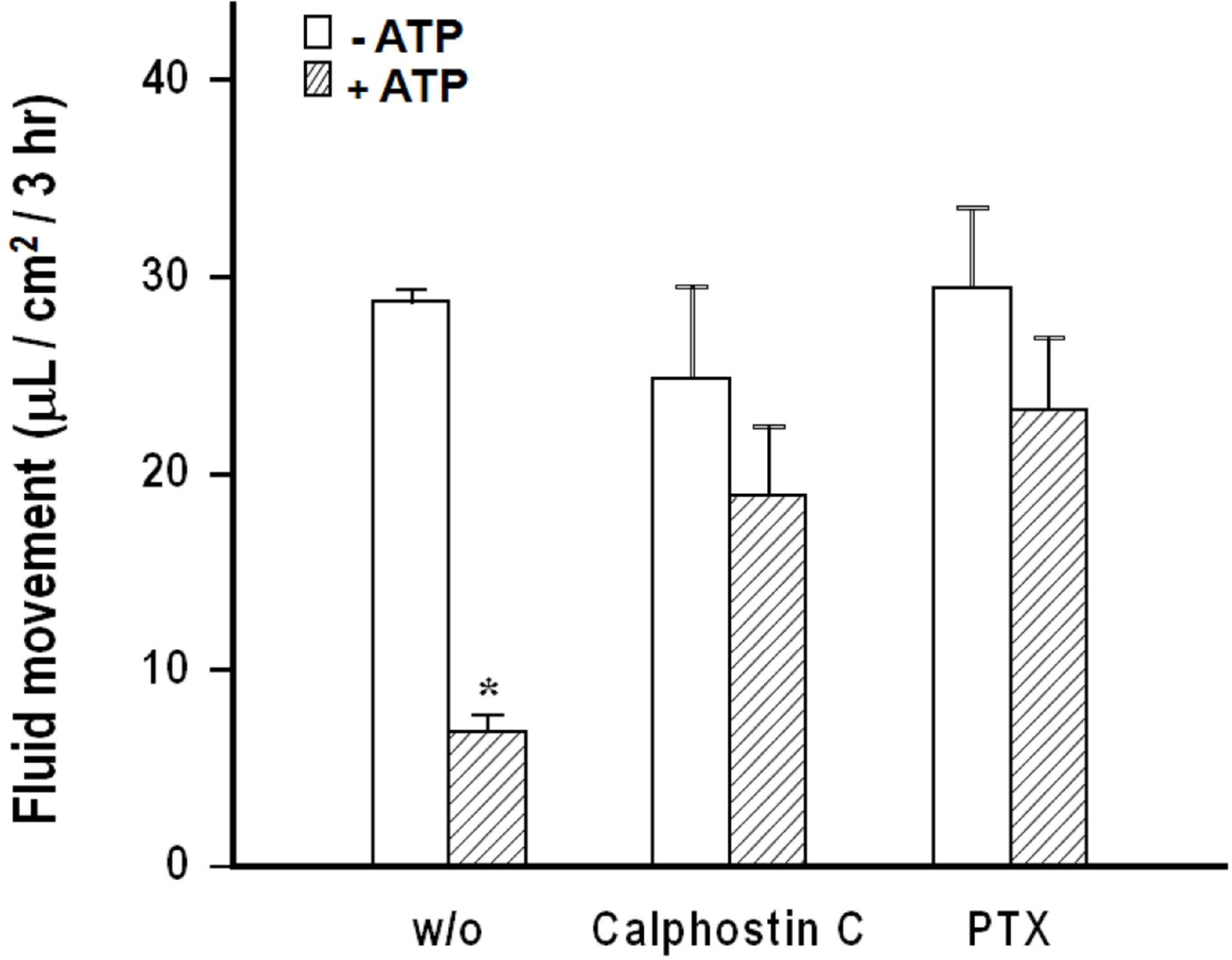
- We cultured canine kidney (MDCK) cells stably expressing aquaporin-2 (AQP2) on collagen-coated permeable membrane filters and examined the effect of extracellular ATP on arginine vasopressin (AVP)-stimulated fluid transport and cAMP production. Exposure of cell monolayers to basolateral AVP resulted in stimulation of apical to basolateral net fluid transport driven by osmotic gradient which was formed by addition of 500 mM mannitol to basolateral bathing solution. Pre-exposure of the basolateral surface of cell monolayers to ATP (100 ?M) for 30 min significantly inhibited the AVP-stimulated net fluid transport. In these cells, AVP-stimulated cAMP production was suppressed as well. Profile of the effects of different nucleotides suggested that the P2Y2 receptor is involved in the action of ATP. ATP inhibited the effect of isoproterenol as well, but not that of forskolin to stimulate cAMP production. The inhibitory effect of ATP on AVP-stimulated fluid movement was attenuated by a protein kinase C inhibitor, calphostin C or pertussis toxin. These results suggest that prolonged activation of the P2 receptors inhibits AVP-stimulated fluid transport and cAMP responses in AQP2 transfected MDCK cells. Depressed responsiveness of the adenylyl cyclase by PKC-mediated modification of the pertussis-toxin sensitive Gi protein seems to be the underlyihng mechanism.
Keyword
MeSH Terms
-
Adenosine Triphosphate
Adenylyl Cyclases
Aquaporin 2
Arginine Vasopressin
Baths
Cyclic AMP
Forskolin
Isoproterenol
Kidney
Madin Darby Canine Kidney Cells
Mannitol
Membranes
Naphthalenes
Nucleotides
Pertussis Toxin
Protein Kinase C
Vasopressins
Adenosine Triphosphate
Aquaporin 2
Arginine Vasopressin
Cyclic AMP
Forskolin
Isoproterenol
Mannitol
Naphthalenes
Nucleotides
Pertussis Toxin
Protein Kinase C
Vasopressins
Figure
Reference
-
Andersen-Beckh B., Dehe M., Schülein R., Wiesner B., Rutz C., Liebenhoff U., Rosenthal W., Oksche A. Polarized expression of the vasopressin V2 receptor in Madin-Darby canine kidney cells. Kidney Int. 56:517–527. 1999.
ArticleBeckner SK., Farrar WL. Inhibition of adenylate cyclase by IL 2 in human T lymphocytes is mediated by protein kinase C. Biochem Biophys Res Commun. 145:176–182. 1987.
ArticleBudnik LT., Mukhopadhyay AK. Desensitisation of LH-stimulated cyclic AMP accumulation in isolated bovine luteal cells-effect of phorbol ester. Mol Cell Endocrinol. 54:51–61. 1987.Buhl AM., Sheikh MI., Steensgaard J., Roigaard-Petersen H., Jacobsen C. GTP-binding proteins in luminal and basolateral membranes from pars convoluta and pars recta of rabbit kidney proximal tubule. FEBS Lett. 304:179–183. 1992.
ArticleDeen PM., Rijss JP., Mulders SM., Errington RJ., Van Baal J., Van Os CH. Aquaporin-2 transfection of Madin–Darby canine kidney cells reconstitutes AVP-regulated transcellular osmotic water transport. J Am Soc Nephrol. 8:1493–1501. 1997.Dubyak GR., El-Moatassim C. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am J Physiol. 265:C577–C606. 1993.
ArticleFredholm BB., Abbracchio MP., Burnstock G., Dubyak GR., Harden TK., Jacobson KA., Schwabe U., Williams M. Towards a revised nomenclature for P1 and P2 receptors. Trends Pharmacol Sci. 18:79–82. 1997.
ArticleHarden TK., Boyer JL., Nicholas RA. P2-purinergic receptors: subtype-associated signaling responses and structure. Annu Rev Pharmacol Toxicol. 35:541–579. 1995.
ArticleHeilbronn E., Knoblauch BH., Muller CE. Uridine nucleotide receptors and their ligands: structural, physiological, and pathophysiological aspects, with special emphasis on the nervous system. Neurochem Res. 22:1041–1050. 1997.Kishore BK., Ginns SM., Krane CM., Nielsen S., Knepper MA. Cellular localization of P2Y2 purinoceptor in rat renal inner medulla and lung. Am J Physiol. 278:F43–F51. 2000.Knepper MA. Molecular physiology of urinary concentrating mechanism: regulation of aquaporin water channels by vasopressin. Am J Physiol. 272:F3–F12. 1997.
ArticleKobayashi E., Nakano H., Morimoto M., Tamaoki T. Calphostin C (UCN-1208C), a novel microbial compound, is a highly potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 159:548–553. 1989.Lin WW., Chuang DD. Endothelin- and ATP-induced inhibition of adenylyl cyclase activity in C6 glioma cells: Role of Gi and calcium. Mol Pharmacol. 44:158–165. 1993.McCoy DE., Taylor AL., Kudlow BA., Karlson K., Slattery MJ., Schwiebert LM., Schwiebert EM., Stanton BA. Nucleotides regulate NaCl transport in mIMCD-K2 cells via P2X and P2Y purinergic receptors. Am J Physiol. 277:F552–F559. 1999.North RA., Barnard EA. Nucleotide receptors. Curr Opin Neurobiol. 7:346–357. 1997.
ArticleOkajima F., Tokumitsu Y., Kondo Y., Ui M. P2-purinergic receptors are coupled to two signal transduction systems leading to inhibition of cAMP generation and to production of inositol triphosphate in rat hepatocytes. J Biol Chem. 262:13483–13490. 1987.Pianet I., Merle M., Labouesse J. ADP and indirectly, ATP are potent inhibitors of cAMP production in intact isoproterenol- stimulated C6 glioma cells. Biochem Biophys Res Commun. 163:1150–1157. 1989.Post SR., Jacobson JP., Insel PA. P2 purinergic receptor agonists enhance cAMP production in Mardin-Darby canine kidney epithelial cells via autocrine/paracrine mechanism. J Biol Chem. 271:2029–2032. 1996.Post SR., Rump LC., Zambon A., Hughes RJ., Buda MD., Jacobson JP., Kao CC., Insel PA. ATP activates cAMP production via multiple purinergic receptors in MDCK-D1 epithelial cells. Blockade of an autocrine/paracrine pathway to define receptor preference of an agonist. J Biol Chem. 273:23093–23097. 1998.Rieg T., Bundey RA., Chen Y., Deschenes G., Junger W., Insel PA., Vallon V. Mice lacking P2Y2 receptors have salt-resistant hypertension and facilitated renal Na+ and water reabsorption. FASEB J. 21:3717–3726. 2007.Sato K., Okajima F., Kondo Y. Extracellular ATP stimulates three different receptor-signal transduction systems in FRTL-5 thyroid cells. Biochem J. 283:282–287. 1992.Teitelbaum I. Protein kinase C inhibits arginine vasopressin-stimulated cAMP accumulation via a Gi-dependent mechanism. Am J Physiol. 264:F216–220. 1993.Vallon V. P2 receptors in the regulation of renal transport mechanisms. Am J Physiol. 294:F10–F27. 2008.
ArticleWilliams M., Burnstock G. Purinergic neurotransmission and neuromodulation: a historical perspective. Jacobson KA, Jarvis MF, editors. ed,. Purinergic Approaches in Experimental Therapeutics. 1st ed.Wiley-Liss, Inc;New York: p. p. 3–26. 1997.Woo JS., Inoue CN., Hanaoka K., Schwiebert EM., Guggino SE., Guggino WB. Adenylyl cyclase is involved in desensitization and recovery of ATP-stimulated Cl- secretion in MDCK cells. Am J Physiol. 274:C371–C378. 1998.Yamada M., Hamamori Y., Akita H., Yokoyama M. P2-purinoceptor activation stimulates phosphoinositide hydrolysis and inhibits accumulation of cAMP in cultured ventricular myocytes. Circ Res. 70:477–485. 1992.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of P2 Nucleotide Receptor Activation on Phosphate Transport in Rabbit Proximal Tubular Cells
- Effect of Diet and Water Intake on Aquaporin 2 Function
- Molecular analysis of AQP2 promoter. I. cAMP-dependent regulation of mouse AQP2 gene
- Regulation of AQP2 in Collecting Duct: An emphasis on the Effects of Angiotensin II or Aldosterone
- A Minireview on Vasopressin-regulated Aquaporin-2 in Kidney Collecting Duct Cells