Cancer Res Treat.
2021 Apr;53(2):567-575. 10.4143/crt.2020.507.
Differing Outcomes of Patients with High Hyperdiploidy and ETV6-RUNX1 Rearrangement in Korean Pediatric Precursor B Cell Acute Lymphoblastic Leukemia
- Affiliations
-
- 1Division of Hematology and Oncology, Department of Pediatrics, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2514937
- DOI: http://doi.org/10.4143/crt.2020.507
Abstract
- Purpose
Recent cooperative trials in pediatric acute lymphoblastic leukemia (ALL) report long-term event-free survival (EFS) of greater than 80%. In this study, we analyzed the outcome and prognostic factors for patients with precursor B cell ALL (n=405) diagnosed during a 10-year period (2005-2015) at our institution.
Materials and Methods
All patients were treated with a uniform institutional regimen based on four risk groups, except for steroid type; patients diagnosed up till 2008 receiving dexamethasone, while subsequent patients received prednisolone. None of the patients received cranial irradiation in first complete remission.
Results
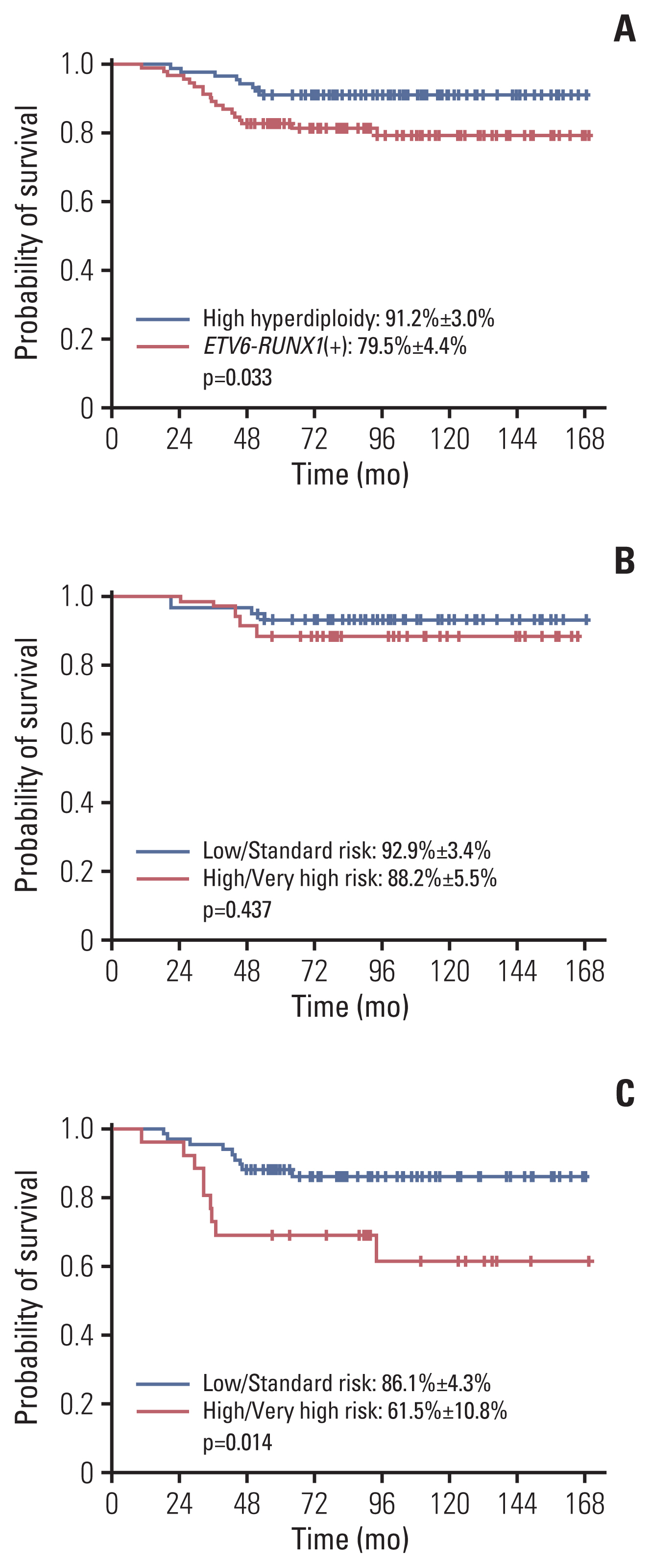
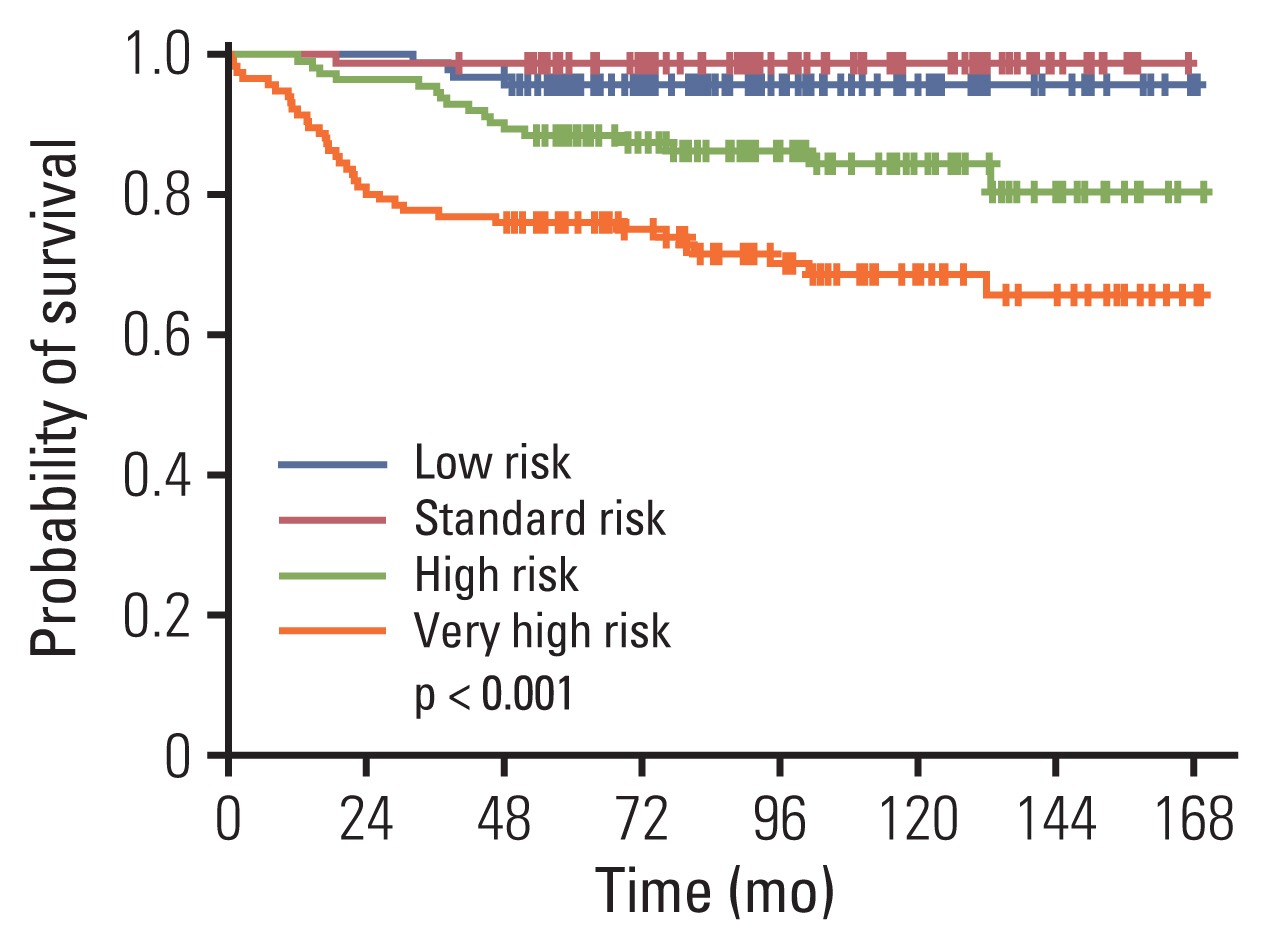
The 10-year EFS and overall survival was 76.3%±2.3% and 85.1%±1.9%. Ten-year cumulative incidence of relapse, any central nervous system (CNS) relapse and isolated CNS relapse was 20.8%±2.2%, 3.7%±1.1% and 2.5%±0.9% respectively. A comparison of established, good prognosis genetic abnormalities showed that patients with high hyperdiploidy had significantly better EFS than those with ETV6-RUNX1 rearrangement (10-year EFS of 91.2%±3.0% vs. 79.5%±4.4%, p=0.033). For the overall cohort, male sex, infant ALL, initial CNS involvement, and Philadelphia chromosome (+) ALL were significant factors for lower EFS in multivariate study, while high hyperdiploidy conferred favorable outcome. For high and very high risk patients (n=231), high hyperdiploidy was the only significant factor for EFS in multivariate study.
Conclusion
Regarding good prognosis genetic abnormalities, patients with high hyperdiploidy had significantly better outcome than ETV6-RUNX1 (+) patients. High hyperdiploidy was a major, favorable prognostic factor in the overall patient group, as well as the subgroup of patients with higher risk.
Figure
Reference
-
References
1. Winter SS, Dunsmore KP, Devidas M, Wood BL, Esiashvili N, Chen Z, et al. Improved survival for children and young adults with T-lineage acute lymphoblastic leukemia: results from the Children’s Oncology Group AALL0434 methotrexate randomization. J Clin Oncol. 2018; 36:2926–34.
Article2. Schramm F, Zur Stadt U, Zimmermann M, Jorch N, Pekrun A, Borkhardt A, et al. Results of CoALL 07-03 study childhood ALL based on combined risk assessment by in vivo and in vitro pharmacosensitivity. Blood Adv. 2019; 3:3688–99.
Article3. Maloney KW, Devidas M, Wang C, Mattano LA, Friedmann AM, Buckley P, et al. Outcome in children with standard-risk B-cell acute lymphoblastic leukemia: results of Children’s Oncology Group Trial AALL0331. J Clin Oncol. 2020; 38:602–12.
Article4. Tubergen DG, Gilchrist GS, O’Brien RT, Coccia PF, Sather HN, Waskerwitz MJ, et al. Improved outcome with delayed intensification for children with acute lymphoblastic leukemia and intermediate presenting features: a Childrens Cancer Group phase III trial. J Clin Oncol. 1993; 11:527–37.
Article5. Reiter A, Schrappe M, Ludwig WD, Hiddemann W, Sauter S, Henze G, et al. Chemotherapy in 998 unselected childhood acute lymphoblastic leukemia patients: results and conclusions of the multicenter trial ALL-BFM 86. Blood. 1994; 84:3122–33.
Article6. Moricke A, Reiter A, Zimmermann M, Gadner H, Stanulla M, Dordelmann M, et al. Risk-adjusted therapy of acute lymphoblastic leukemia can decrease treatment burden and improve survival: treatment results of 2169 unselected pediatric and adolescent patients enrolled in the trial ALL-BFM 95. Blood. 2008; 111:4477–89.7. Moricke A, Zimmermann M, Reiter A, Henze G, Schrauder A, Gadner H, et al. Long-term results of five consecutive trials in childhood acute lymphoblastic leukemia performed by the ALL-BFM study group from 1981 to 2000. Leukemia. 2010; 24:265–84.
Article8. Veerman AJ, Kamps WA, van den Berg H, van den Berg E, Bokkerink JP, Bruin MC, et al. Dexamethasone-based therapy for childhood acute lymphoblastic leukaemia: results of the prospective Dutch Childhood Oncology Group (DCOG) protocol ALL-9 (1997–2004). Lancet Oncol. 2009; 10:957–66.
Article9. Jeha S, Pei D, Choi J, Cheng C, Sandlund JT, Coustan-Smith E, et al. Improved CNS control of childhood acute lymphoblastic leukemia without cranial irradiation: St Jude Total Therapy Study 16. J Clin Oncol. 2019; 37:3377–91.
Article10. Toft N, Birgens H, Abrahamsson J, Griskevicius L, Hallbook H, Heyman M, et al. Results of NOPHO ALL2008 treatment for patients aged 1–45 years with acute lymphoblastic leukemia. Leukemia. 2018; 32:606–15.
Article11. Attarbaschi A, Mann G, Zimmermann M, Bader P, Barisone E, Basso G, et al. Randomized post-induction and delayed intensification therapy in high-risk pediatric acute lymphoblastic leukemia: long-term results of the international AIEOP-BFM ALL 2000 trial. Leukemia. 2020; 34:1694–700.
Article12. Moorman AV, Ensor HM, Richards SM, Chilton L, Schwab C, Kinsey SE, et al. Prognostic effect of chromosomal abnormalities in childhood B-cell precursor acute lymphoblastic leukaemia: results from the UK Medical Research Council ALL97/99 randomised trial. Lancet Oncol. 2010; 11:429–38.
Article13. Lee JW, Kim SK, Jang PS, Jeong DC, Chung NG, Cho B, et al. Treatment of children with acute lymphoblastic leukemia with risk group based intensification and omission of cranial irradiation: a Korean study of 295 patients. Pediatr Blood Cancer. 2016; 63:1966–73.
Article14. Shin J, Lee NY, Kim S, Lee JW, Jang PS, Chung NG, et al. Outcome and prognostic factors of children with Philadelphia chromosome-positive acute lymphoblastic leukemia treated with imatinib followed by allogeneic hematopoietic cell trans-plantation in first remission. Blood Res. 2019; 54:45–51.
Article15. Vora A, Goulden N, Wade R, Mitchell C, Hancock J, Hough R, et al. Treatment reduction for children and young adults with low-risk acute lymphoblastic leukaemia defined by minimal residual disease (UKALL 2003): a randomised controlled trial. Lancet Oncol. 2013; 14:199–209.
Article16. Vora A, Goulden N, Mitchell C, Hancock J, Hough R, Rowntree C, et al. Augmented post-remission therapy for a minimal residual disease-defined high-risk subgroup of children and young people with clinical standard-risk and intermediate-risk acute lymphoblastic leukaemia (UKALL 2003): a randomised controlled trial. Lancet Oncol. 2014; 15:809–18.
Article17. Nagayama J, Tomizawa D, Koh K, Nagatoshi Y, Hotta N, Kishimoto T, et al. Infants with acute lymphoblastic leukemia and a germline MLL gene are highly curable with use of chemotherapy alone: results from the Japan Infant Leukemia Study Group. Blood. 2006; 107:4663–5.
Article18. Pieters R, De Lorenzo P, Ancliffe P, Aversa LA, Brethon B, Biondi A, et al. Outcome of infants younger than 1 year with acute lymphoblastic leukemia treated with the Interfant-06 Protocol: results from an international phase III randomized study. J Clin Oncol. 2019; 37:2246–56.
Article19. Koh K, Tomizawa D, Moriya Saito A, Watanabe T, Miyamura T, Hirayama M, et al. Early use of allogeneic hematopoietic stem cell transplantation for infants with MLL gene-rearrangement-positive acute lymphoblastic leukemia. Leukemia. 2015; 29:290–6.
Article20. Clesham K, Rao V, Bartram J, Ancliff P, Ghorashian S, O’Connor D, et al. Blinatumomab for infant acute lymphoblastic leukemia. Blood. 2020; 135:1501–4.
Article21. Hasegawa D, Imamura T, Yumura-Yagi K, Takahashi Y, Usami I, Suenobu SI, et al. Risk-adjusted therapy for pediatric non-T cell ALL improves outcomes for standard risk patients: results of JACLS ALL-02. Blood Cancer J. 2020; 10:23.
Article22. Schultz KR, Carroll A, Heerema NA, Bowman WP, Aledo A, Slayton WB, et al. Long-term follow-up of imatinib in pediatric Philadelphia chromosome-positive acute lymphoblastic leukemia: Children’s Oncology Group study AALL0031. Leukemia. 2014; 28:1467–71.
Article23. Slayton WB, Schultz KR, Kairalla JA, Devidas M, Mi X, Pulsipher MA, et al. Dasatinib plus intensive chemotherapy in children, adolescents, and young adults with Philadelphia chromosome-positive acute lymphoblastic leukemia: results of Children’s Oncology Group Trial AALL0622. J Clin Oncol. 2018; 36:2306–14.
Article24. Moorman AV, Richards SM, Martineau M, Cheung KL, Robinson HM, Jalali GR, et al. Outcome heterogeneity in childhood high-hyperdiploid acute lymphoblastic leukemia. Blood. 2003; 102:2756–62.
Article25. Reismuller B, Steiner M, Pichler H, Dworzak M, Urban C, Meister B, et al. High hyperdiploid acute lymphoblastic leukemia (ALL): a 25-year population-based survey of the Austrian ALL-BFM (Berlin-Frankfurt-Munster) Study Group. Pediatr Blood Cancer. 2017; 64:e26327.26. Arico M, Valsecchi MG, Rizzari C, Barisone E, Biondi A, Casale F, et al. Long-term results of the AIEOP-ALL-95 Trial for Childhood Acute Lymphoblastic Leukemia: insight on the prognostic value of DNA index in the framework of Berlin-Frankfurt-Muenster based chemotherapy. J Clin Oncol. 2008; 26:283–9.27. Madzo J, Zuna J, Muzikova K, Kalinova M, Krejci O, Hrusak O, et al. Slower molecular response to treatment predicts poor outcome in patients with TEL/AML1 positive acute lymphoblastic leukemia: prospective real-time quantitative reverse transcriptase-polymerase chain reaction study. Cancer. 2003; 97:105–13.28. Ampatzidou M, Papadhimitriou SI, Paterakis G, Pavlidis D, Tsitsikas K, Kostopoulos IV, et al. ETV6/RUNX1-positive childhood acute lymphoblastic leukemia (ALL): The spectrum of clonal heterogeneity and its impact on prognosis. Cancer Genet. 2018; 224–225:1–11.
Article29. Lee JW, Kim SK, Jang PS, Chung NG, Jeong DC, Kim M, et al. Outcome and prognostic factors for ETV6/RUNX1 positive pediatric acute lymphoblastic leukemia treated at a single institution in Korea. Cancer Res Treat. 2017; 49:446–53.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Outcome and Prognostic Factors for ETV6/RUNX1 Positive Pediatric Acute Lymphoblastic Leukemia Treated at a Single Institution in Korea
- Precursor B-Cell Acute Lymphoblastic Leukemia in Two Patients with a History of Cytotoxic Therapy
- A Case of CD7+, CD4-, CD8-, CD3-acute T cell lymphoblastic leukemia
- Mixed-phenotypic acute leukemia: cytochemically myeloid and phenotypically early T-cell precursor acute lymphoblastic leukemia
- A Case of Pediatric Acute Lymphoblastic Leukemia with Trisomy 5 as a Sole Chromosomal Anomaly: A Prognostic Significance