Metagenomic Analysis of the Gut Microbiome Reveals Enrichment of Menaquinones (Vitamin K2) Pathway in Diabetes Mellitus
- Affiliations
-
- 1Department of Clinical Sciences, College of Medicine, University of Sharjah, Sharjah, United Arab Emirates.
- 2Research Institute for Medical & Health Sciences at University of Sharjah, Sharjah, United Arab Emirates.
- KMID: 2514076
- DOI: http://doi.org/10.4093/dmj.2019.0202
Abstract
Background Type 2 diabetes mellitus (T2DM) is a chronic metabolic disease with a high prevalence worldwide, especially among overweight and obese populations. T2DM is multifactorial with several genetic and acquired risk factors that lead to insulin resistance. Mounting evidence indicates that alteration of gut microbiome composition contribute to insulin resistance and inflammation. However, the precise link between T2DM and gut microbiome role and composition remains unknown.
Methods We evaluated the metabolic capabilities of the gut microbiome of twelve T2DM and six healthy individuals through shotgun metagenomics using MiSeq platform.
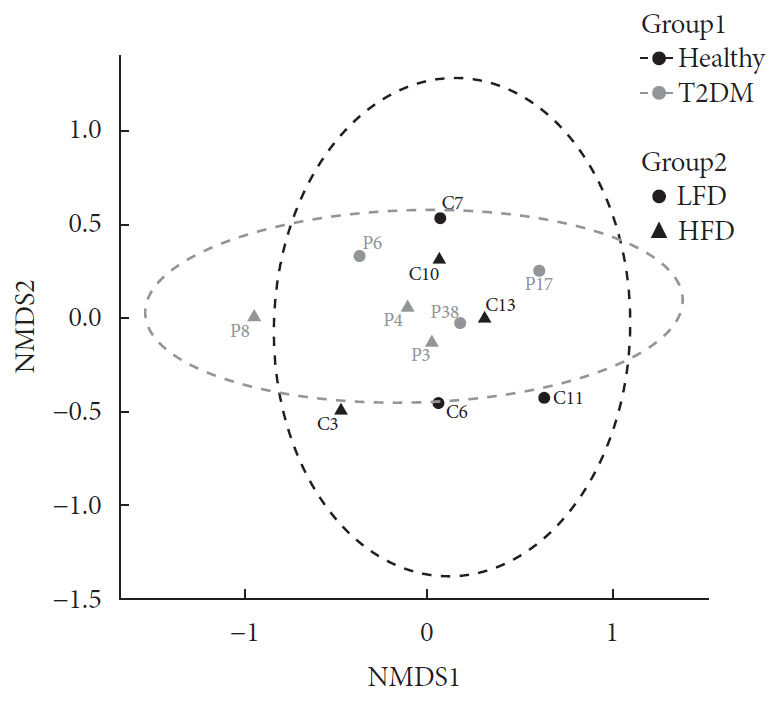
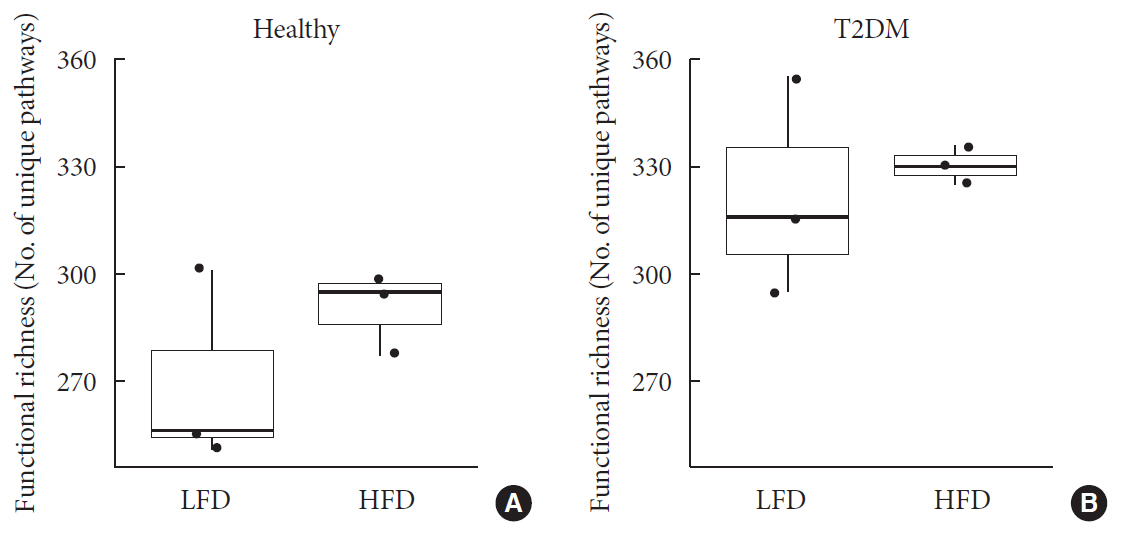
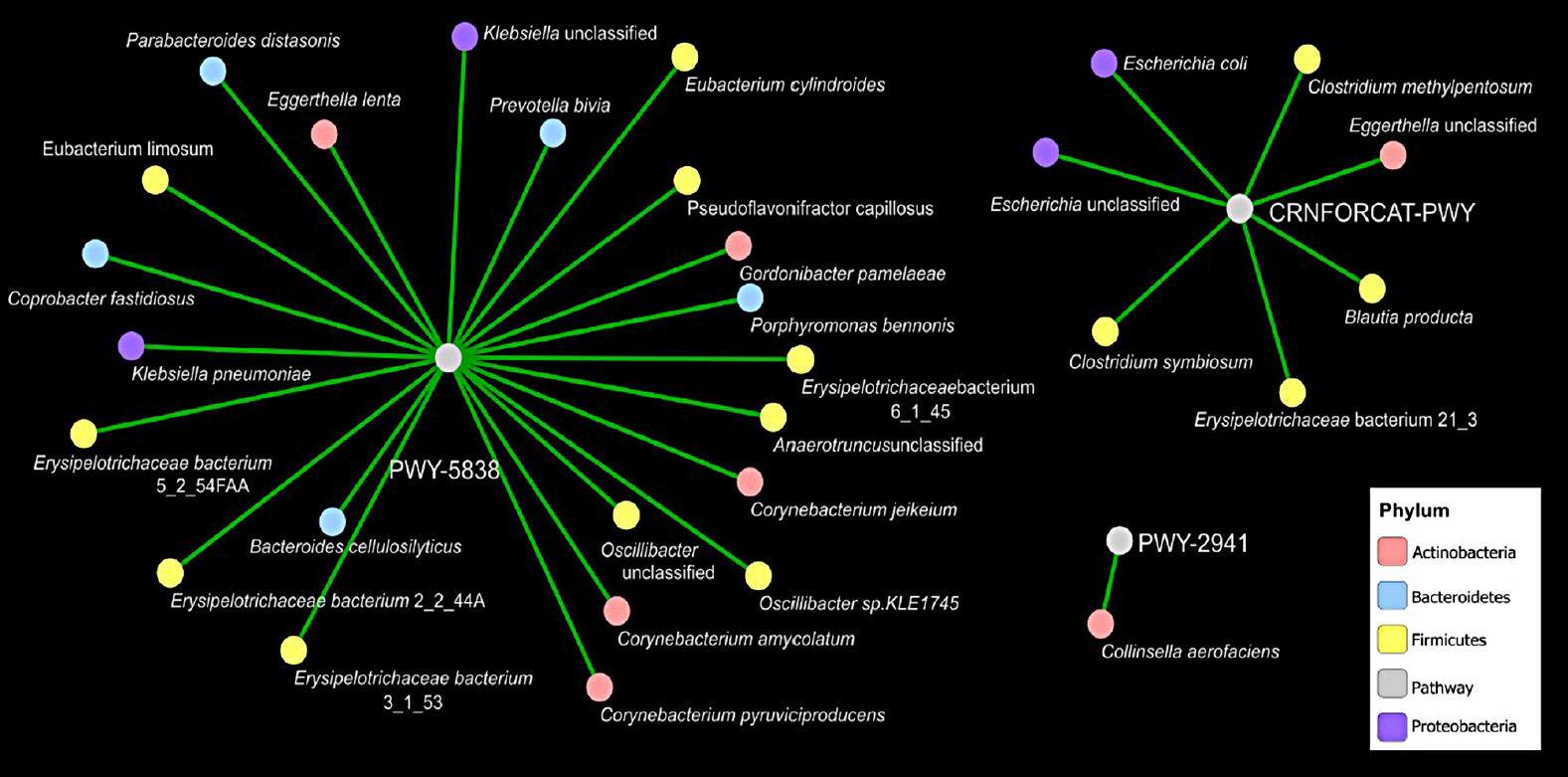
Results We identified no significant differences in the overall taxonomic composition between healthy and T2DM subjects when controlling for differences in diet. However, results showed that T2DM enriched in metabolic pathways involved in menaquinone (vitamin K2) superpathway biosynthesis (PWY-5838) as compared to healthy individuals. Covariance analysis between the bacterial genera and metabolic pathways displaying difference in abundance (analysis of variance
P <0.05) in T2DM as compared to healthy subjects revealed that genera belongingFirmicutes ,Actinobacteria , andBacteroidetes phyla contribute significantly to vitamin K2 biosynthesis. Further, the microbiome corresponding to T2DM with high glycosylated hemoglobin (HbA1c) (>6.5%) exhibit high abundance of genes involved in lysine biosynthesis and low abundance of genes involved in creatinine degradation as compared to T2DM with lower HbA1c (<6.5%).Conclusion The identified differences in metabolic capabilities provide important information that may eventually lead to the development of novel biomarkers and more effective management strategies to treat T2DM.
Keyword
Figure
Reference
-
1. Tilg H, Moschen AR. Microbiota and diabetes: an evolving relationship. Gut. 2014; 63:1513–1521.
Article2. Larsen N, Vogensen FK, van den Berg FW, Nielsen DS, Andreasen AS, Pedersen BK, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One. 2010; 5:e9085.
Article3. Zhang X, Shen D, Fang Z, Jie Z, Qiu X, Zhang C, et al. Human gut microbiota changes reveal the progression of glucose intolerance. PLoS One. 2013; 8:e71108.
Article4. Horton F, Wright J, Smith L, Hinton PJ, Robertson MD. Increased intestinal permeability to oral chromium (51 Cr): EDTA in human type 2 diabetes. Diabet Med. 2014; 31:559–563.5. Cani PD, Geurts L, Matamoros S, Plovier H, Duparc T. Glucose metabolism: focus on gut microbiota, the endocannabinoid system and beyond. Diabetes Metab. 2014; 40:246–257.
Article6. Joice R, Yasuda K, Shafquat A, Morgan XC, Huttenhower C. Determining microbial products and identifying molecular targets in the human microbiome. Cell Metab. 2014; 20:731–741.
Article7. Langille MG, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol. 2013; 31:814–821.
Article8. Knight R, Jansson J, Field D, Fierer N, Desai N, Fuhrman JA, et al. Unlocking the potential of metagenomics through replicated experimental design. Nat Biotechnol. 2012; 30:513–520.
Article9. Vital M, Howe AC, Tiedje JM. Revealing the bacterial butyrate synthesis pathways by analyzing (meta)genomic data. mBio. 2014; 5:e00889.
Article10. Levy R, Borenstein E. Metagenomic systems biology and metabolic modeling of the human microbiome: from species composition to community assembly rules. Gut Microbes. 2014; 5:265–270.11. Pimentel M, Mathur R, Chang C. Gas and the microbiome. Curr Gastroenterol Rep. 2013; 15:356.
Article12. Gundberg CM, Markowitz ME, Mizruchi M, Rosen JF. Osteocalcin in human serum: a circadian rhythm. J Clin Endocrinol Metab. 1985; 60:736–739.
Article13. Knapen MH, Braam LA, Drummen NE, Bekers O, Hoeks AP, Vermeer C. Menaquinone-7 supplementation improves arterial stiffness in healthy postmenopausal women: a double-blind randomised clinical trial. Thromb Haemost. 2015; 113:1135–1144.14. Beulens JW, van der A DL, Grobbee DE, Sluijs I, Spijkerman AM, van der Schouw YT. Dietary phylloquinone and menaquinones intakes and risk of type 2 diabetes. Diabetes Care. 2010; 33:1699–1705.
Article15. Choi HJ, Yu J, Choi H, An JH, Kim SW, Park KS, et al. Vitamin K2 supplementation improves insulin sensitivity via osteocalcin metabolism: a placebo-controlled trial. Diabetes Care. 2011; 34:e147.
Article16. Healey G, Brough L, Murphy R, Hedderley D, Butts C, Coad J. Validity and reproducibility of a habitual dietary fibre intake short food frequency questionnaire. Nutrients. 2016; 8:E558.
Article17. Magoc T, Salzberg SL. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011; 27:2957–2963.
Article18. Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014; 30:2114–2120.
Article19. Caspi R, Billington R, Ferrer L, Foerster H, Fulcher CA, Keseler IM, et al. The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of pathway/genome databases. Nucleic Acids Res. 2016; 44:D471–D480.
Article20. Truong DT, Franzosa EA, Tickle TL, Scholz M, Weingart G, Pasolli E, et al. MetaPhlAn2 for enhanced metagenomic taxonomic profiling. Nat Methods. 2015; 12:902–903.
Article21. Claesson MJ, Jeffery IB, Conde S, Power SE, O'Connor EM, Cusack S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012; 488:178–184.
Article22. Saraswati S, Sitaraman R. Aging and the human gut microbiota-from correlation to causality. Front Microbiol. 2015; 5:764.
Article23. Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012; 490:55–60.24. Wang J, Jia H. Metagenome-wide association studies: fine-mining the microbiome. Nat Rev Microbiol. 2016; 14:508–522.
Article25. Abul-Hajj YJ. Isolation of vitamin K2(35) from Nocardia restrictus and Corynebacterium simplex: a natural electron acceptor in microbial steroid ring A dehydrogenations. J Biol Chem. 1978; 253:2356–2360.
Article26. Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, et al. Human gut microbiome viewed across age and geography. Nature. 2012; 486:222–227.
Article27. Tremaroli V, Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012; 489:242–249.
Article28. An R, Wilms E, Masclee AAM, Smidt H, Zoetendal EG, Jonkers D. Age-dependent changes in GI physiology and microbiota: time to reconsider. Gut. 2018; 67:2213–2222.
Article29. Fransen F, van Beek AA, Borghuis T, Meijer B, Hugenholtz F, van der Gaast-de Jongh C, et al. The impact of gut microbiota on gender-specific differences in immunity. Front Immunol. 2017; 8:754.
Article30. Forslund K, Hildebrand F, Nielsen T, Falony G, Le Chatelier E, Sunagawa S, et al. MetaHIT consortium. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015; 528:262–266.
Article31. Cani PD, Osto M, Geurts L, Everard A. Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes. 2012; 3:279–288.
Article32. Chassaing B, Gewirtz AT. Gut microbiota, low-grade inflammation, and metabolic syndrome. Toxicol Pathol. 2014; 42:49–53.
Article33. Conly JM, Stein K. The production of menaquinones (vitamin K2) by intestinal bacteria and their role in maintaining coagulation homeostasis. Prog Food Nutr Sci. 1992; 16:307–343.34. Suttie JW. The importance of menaquinones in human nutrition. Annu Rev Nutr. 1995; 15:399–417.
Article35. Yoshida M, Jacques PF, Meigs JB, Saltzman E, Shea MK, Gundberg C, et al. Effect of vitamin K supplementation on insulin resistance in older men and women. Diabetes Care. 2008; 31:2092–2096.
Article36. Yamaguchi M, Weitzmann MN. Vitamin K2 stimulates osteoblastogenesis and suppresses osteoclastogenesis by suppressing NF-κB activation. Int J Mol Med. 2011; 27:3–14.
Article37. Thakore AH, Guo CY, Larson MG, Corey D, Wang TJ, Vasan RS, et al. Association of multiple inflammatory markers with carotid intimal medial thickness and stenosis (from the Framingham Heart Study). Am J Cardiol. 2007; 99:1598–1602.
Article38. Sakamoto N, Nishiike T, Iguchi H, Sakamoto K. Possible effects of one week vitamin K (menaquinone-4) tablets intake on glucose tolerance in healthy young male volunteers with different descarboxy prothrombin levels. Clin Nutr. 2000; 19:259–263.
Article39. Palm NW, de Zoete MR, Cullen TW, Barry NA, Stefanowski J, Hao L, et al. Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell. 2014; 158:1000–1010.
Article40. Dinh DM, Volpe GE, Duffalo C, Bhalchandra S, Tai AK, Kane AV, et al. Intestinal microbiota, microbial translocation, and systemic inflammation in chronic HIV infection. J Infect Dis. 2015; 211:19–27.
Article41. Schaubeck M, Clavel T, Calasan J, Lagkouvardos I, Haange SB, Jehmlich N, et al. Dysbiotic gut microbiota causes transmissible Crohn's disease-like ileitis independent of failure in antimicrobial defence. Gut. 2016; 65:225–237.
Article42. Zhang H, DiBaise JK, Zuccolo A, Kudrna D, Braidotti M, Yu Y, et al. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci U S A. 2009; 106:2365–2370.
Article43. Kosanam H, Thai K, Zhang Y, Advani A, Connelly KA, Diamandis EP, et al. Diabetes induces lysine acetylation of intermediary metabolism enzymes in the kidney. Diabetes. 2014; 63:2432–2439.
Article44. Jones JD, Burnett PC. Creatinine metabolism in humans with decreased renal function: creatinine deficit. Clin Chem. 1974; 20:1204–1212.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Microbiome and Diabetes
- Does bisphenol-A affect alteration of gut microbiome after bariatric/metabolic surgery?: a comparative metagenomic analysis in a long-term high-fat diet induced-obesity rat model
- Subgingival microbiome in periodontitis and type 2 diabetes mellitus: an exploratory study using metagenomic sequencing
- Diabetes and Vitamin D
- Vitamin D and Diabetes Mellitus