Ann Surg Treat Res.
2022 Jun;102(6):342-352. 10.4174/astr.2022.102.6.342.
Does bisphenol-A affect alteration of gut microbiome after bariatric/metabolic surgery?: a comparative metagenomic analysis in a long-term high-fat diet induced-obesity rat model
- Affiliations
-
- 1Department of Surgery, Ajou University School of Medicine, Suwon, Korea
- 2Department of Gastrointestinal Surgery, The Second Affiliated Hospital of Dalian Medical University, Dalian, China
- 3Department of Surgery, Seoul National University Bundang Hospital, Seongnam, Korea
- KMID: 2530389
- DOI: http://doi.org/10.4174/astr.2022.102.6.342
Abstract
- Purpose
Bisphenol A (BPA) is a widely used environmental contaminant that is associated with type 2 diabetes mellitus and a shift of gut microbial community. However, little is known about the influence of BPA on gut microbial changes related to bariatric surgery. We investigated whether long-term exposure to dietary BPA causing alterations of gut microbiome occurred after bariatric surgery.
Methods
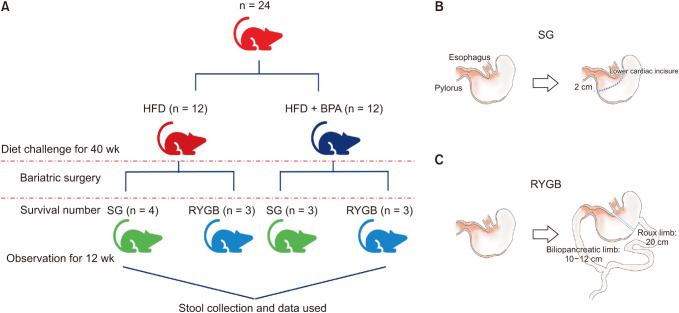
Six-week-old male Wistar rats were fed either a high- fat diet (HFD) or HFD + BPA for 40 weeks. Then sleeve gastrectomy (SG) or Roux-en Y gastric bypass (RYGB) was performed in each diet group and observed for 12 weeks postoperatively. Fecal samples were collected at the 40th weeks and 12th postoperative weeks. Using 16S ribosomal RNA gene sequencing analysis on fecal samples, a comparative metagenomic analysis on gut microbiome composition was performed.
Results
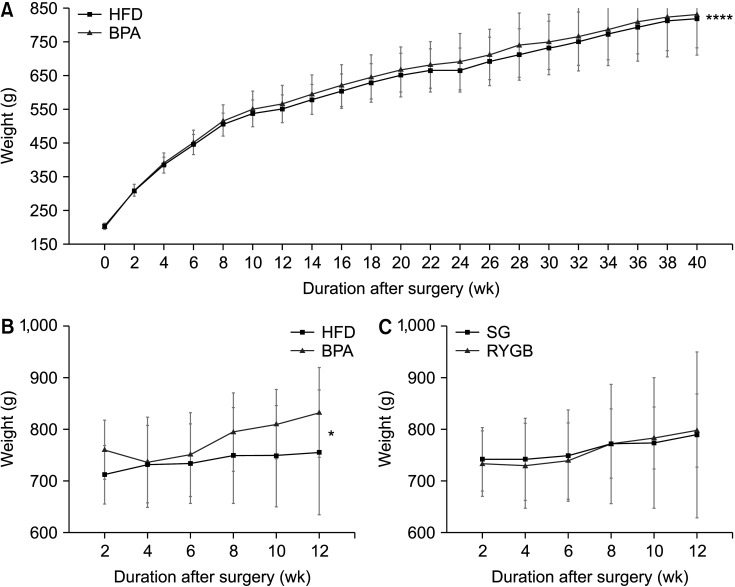
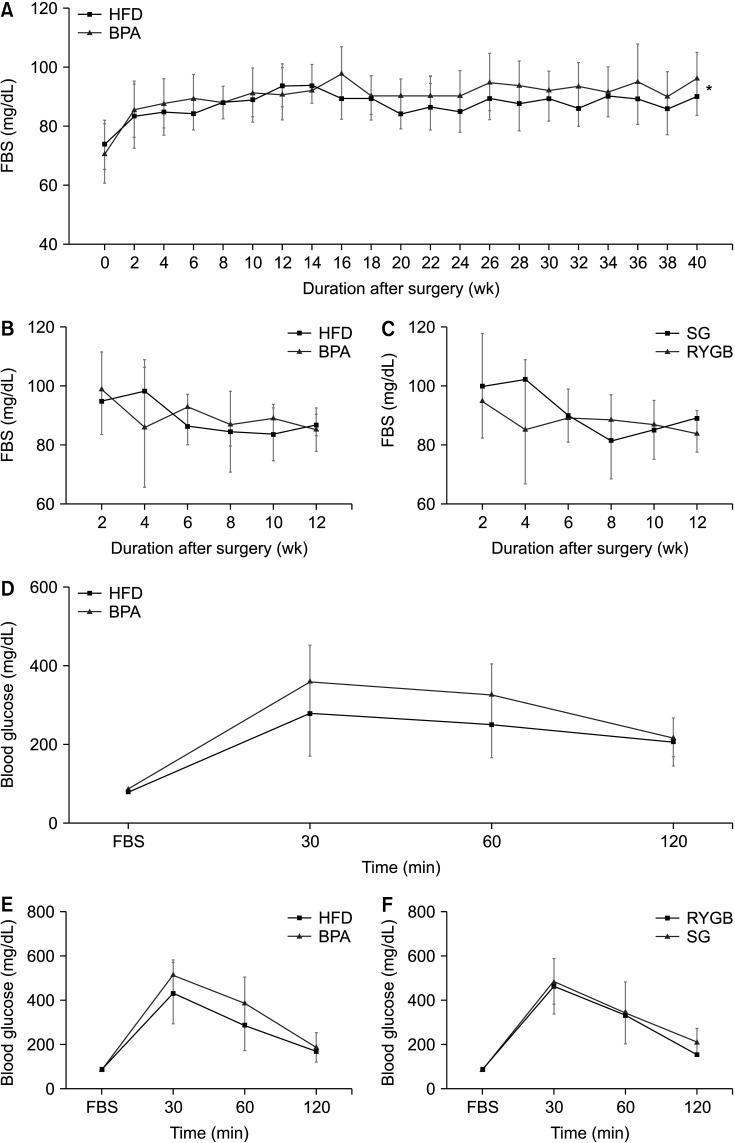
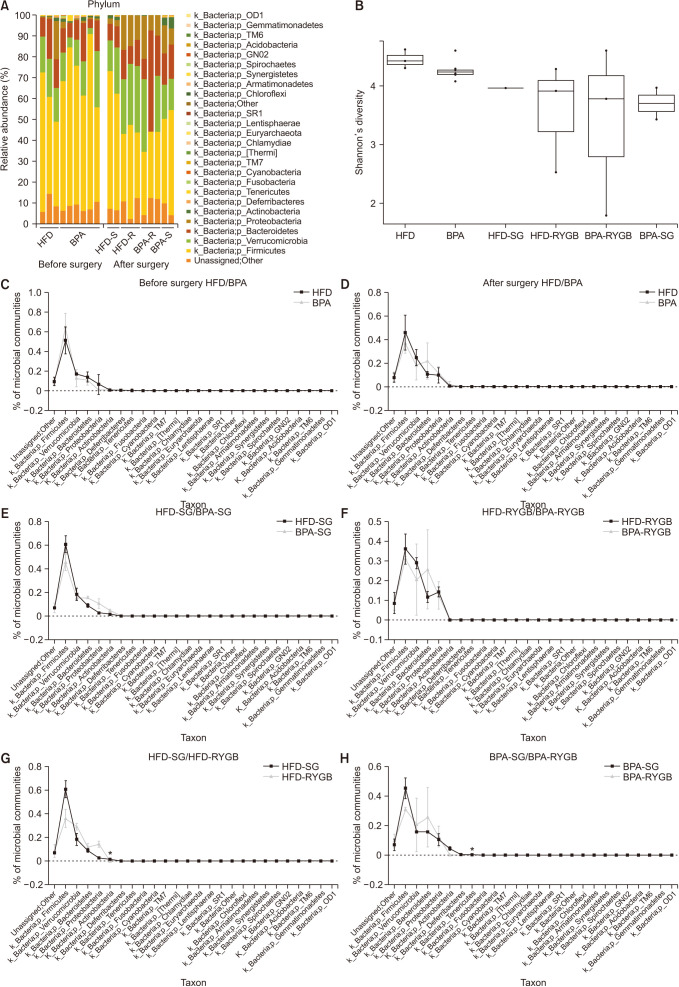
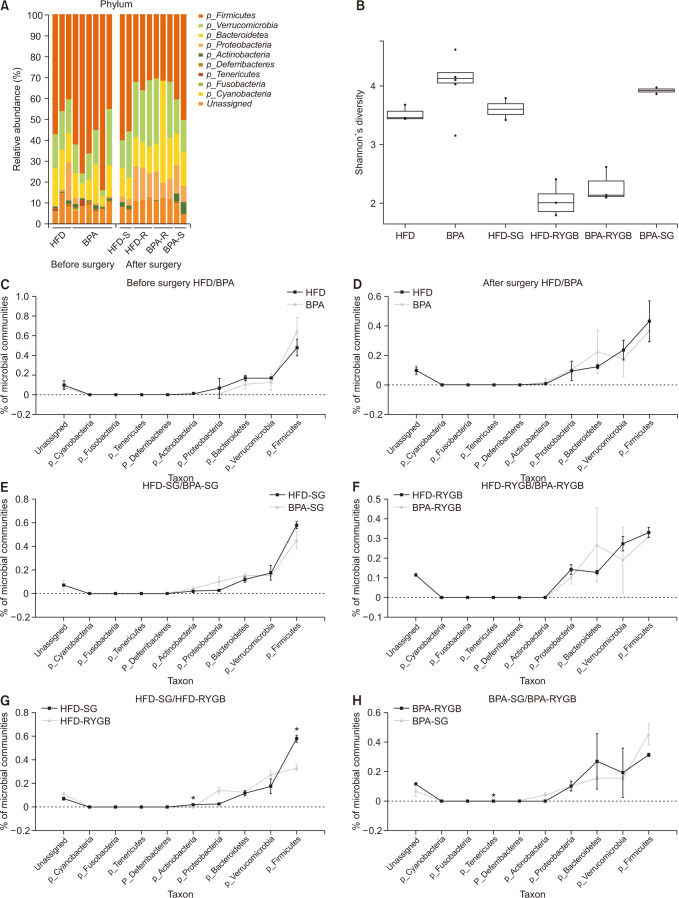
Long-term exposure to HFD with BPA showed higher body weight change and higher level of fasting blood sugar after 40 weeks-diet challenge than those of the HFD only group. After bariatric surgeries, mean body weight of the HFD with BPA group was significantly higher than the HFD only group, but there was no difference between the SG and RYGB groups. The metagenomic analyses demonstrated that long-term exposure to dietary BPA did not affect significant alterations of gut microbiome before and after bariatric surgery, compared with the HFD groups.
Conclusion
Our results highlighted that BPA was a risk factor for obesity and may contribute to glucose intolerance, but it did not affect alterations of gut microbiome after bariatric/metabolic surgery.
Figure
Reference
-
1. Cani PD, Osto M, Geurts L, Everard A. Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes. 2012; 3:279–288. PMID: 22572877.
Article2. Schwiertz A, Taras D, Schäfer K, Beijer S, Bos NA, Donus C, et al. Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring). 2010; 18:190–195. PMID: 19498350.
Article3. Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010; 90:859–904. PMID: 20664075.
Article4. Guo Y, Huang ZP, Liu CQ, Qi L, Sheng Y, Zou DJ. Modulation of the gut microbiome: a systematic review of the effect of bariatric surgery. Eur J Endocrinol. 2018; 178:43–56. PMID: 28916564.
Article5. Ciobârcă D, Cătoi AF, Copăescu C, Miere D, Crișan G. Bariatric surgery in obesity: effects on gut microbiota and micronutrient status. Nutrients. 2020; 12:235.
Article6. Geens T, Aerts D, Berthot C, Bourguignon JP, Goeyens L, Lecomte P, et al. A review of dietary and non-dietary exposure to bisphenol-A. Food Chem Toxicol. 2012; 50:3725–3740. PMID: 22889897.
Article7. Cao XL, Perez-Locas C, Dufresne G, Clement G, Popovic S, Beraldin F, et al. Concentrations of bisphenol A in the composite food samples from the 2008 Canadian total diet study in Quebec City and dietary intake estimates. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2011; 28:791–798. PMID: 21623504.
Article8. Matthews JB, Twomey K, Zacharewski TR. In vitro and in vivo interactions of bisphenol A and its metabolite, bisphenol A glucuronide, with estrogen receptors alpha and beta. Chem Res Toxicol. 2001; 14:149–157. PMID: 11258963.
Article9. Alonso-Magdalena P, Quesada I, Nadal A. Endocrine disruptors in the etiology of type 2 diabetes mellitus. Nat Rev Endocrinol. 2011; 7:346–353. PMID: 21467970.
Article10. Do MT, Chang VC, Mendez MA, de Groh M. Urinary bisphenol A and obesity in adults: results from the Canadian Health Measures Survey. Health Promot Chronic Dis Prev Can. 2017; 37:403–412. PMID: 29236378.
Article11. Lang IA, Galloway TS, Scarlett A, Henley WE, Depledge M, Wallace RB, et al. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA. 2008; 300:1303–1310. PMID: 18799442.
Article12. Shankar A, Teppala S. Relationship between urinary bisphenol A levels and diabetes mellitus. J Clin Endocrinol Metab. 2011; 96:3822–3826. PMID: 21956417.
Article13. Provvisiero DP, Pivonello C, Muscogiuri G, Negri M, de Angelis C, Simeoli C, et al. Influence of bisphenol A on type 2 diabetes mellitus. Int J Environ Res Public Health. 2016; 13:989.
Article14. Lai KP, Chung YT, Li R, Wan HT, Wong CK. Bisphenol A alters gut microbiome: comparative metagenomics analysis. Environ Pollut. 2016; 218:923–930. PMID: 27554980.
Article15. Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011; 17:10–12.
Article16. Bokulich NA, Subramanian S, Faith JJ, Gevers D, Gordon JI, Knight R, et al. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat Methods. 2013; 10:57–59. PMID: 23202435.
Article17. Kwon S, Lee B, Yoon S. CASPER: context-aware scheme for paired-end reads from high-throughput amplicon sequencing. BMC Bioinformatics. 2014; 15(Suppl 9):S10.
Article18. Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013; 41(Database issue):D590–D596. PMID: 23193283.
Article19. Rognes T, Flouri T, Nichols B, Quince C, Mahé F. VSEARCH: a versatile open source tool for metagenomics. PeerJ. 2016; 4:e2584. PMID: 27781170.
Article20. Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010; 7:335–336. PMID: 20383131.
Article21. Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, et al. EDC-2: The Endocrine Society’s second scientific statement on endocrine-disrupting chemicals. Endocr Rev. 2015; 36:E1–E150. PMID: 26544531.
Article22. Heindel J J, Blumberg B, Cave M, Machtinger R, Mantovani A, Mendez MA, et al. Metabolism disrupting chemicals and metabolic disorders. Reprod Toxicol. 2017; 68:3–33. PMID: 27760374.
Article23. Rubin BS, Soto AM. Bisphenol A: perinatal exposure and body weight. Mol Cell Endocrinol. 2009; 304:55–62. PMID: 19433248.
Article24. Wang HX, Zhou Y, Tang CX, Wu JG, Chen Y, Jiang QW. Association between bisphenol A exposure and body mass index in Chinese school children: a cross-sectional study. Environ Health. 2012; 11:79. PMID: 23083070.
Article25. Alonso-Magdalena P, Vieira E, Soriano S, Menes L, Burks D, Quesada I, et al. Bisphenol A exposure during pregnancy disrupts glucose homeostasis in mothers and adult male offspring. Environ Health Perspect. 2010; 118:1243–1250. PMID: 20488778.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Alterations in Gut Microbiota and Immunity by Dietary Fat
- Perioperative Nutritional Management of Morbid Obesity
- 2018 Korean Society for Metabolic and Bariatric Surgery Guidelines
- Effect of Diet on the Gut Microbiota Associated with Obesity
- Current Issues in Bariatric Surgery for Adolescents with Severe Obesity: Durability, Complications, and Timing of Intervention