J Bacteriol Virol.
2020 Dec;50(4):263-272. 10.4167/jbv.2020.50.4.263.
Isolation and Molecular Characterization of Feline Herpesvirus 1 from Naturally Infected Korean Cats
- Affiliations
-
- 1Viral Disease Research Division, Animal and Plant Quarantine Agency, MAFRA, Gimcheon 39660, Republic of Korea
- KMID: 2512146
- DOI: http://doi.org/10.4167/jbv.2020.50.4.263
Abstract
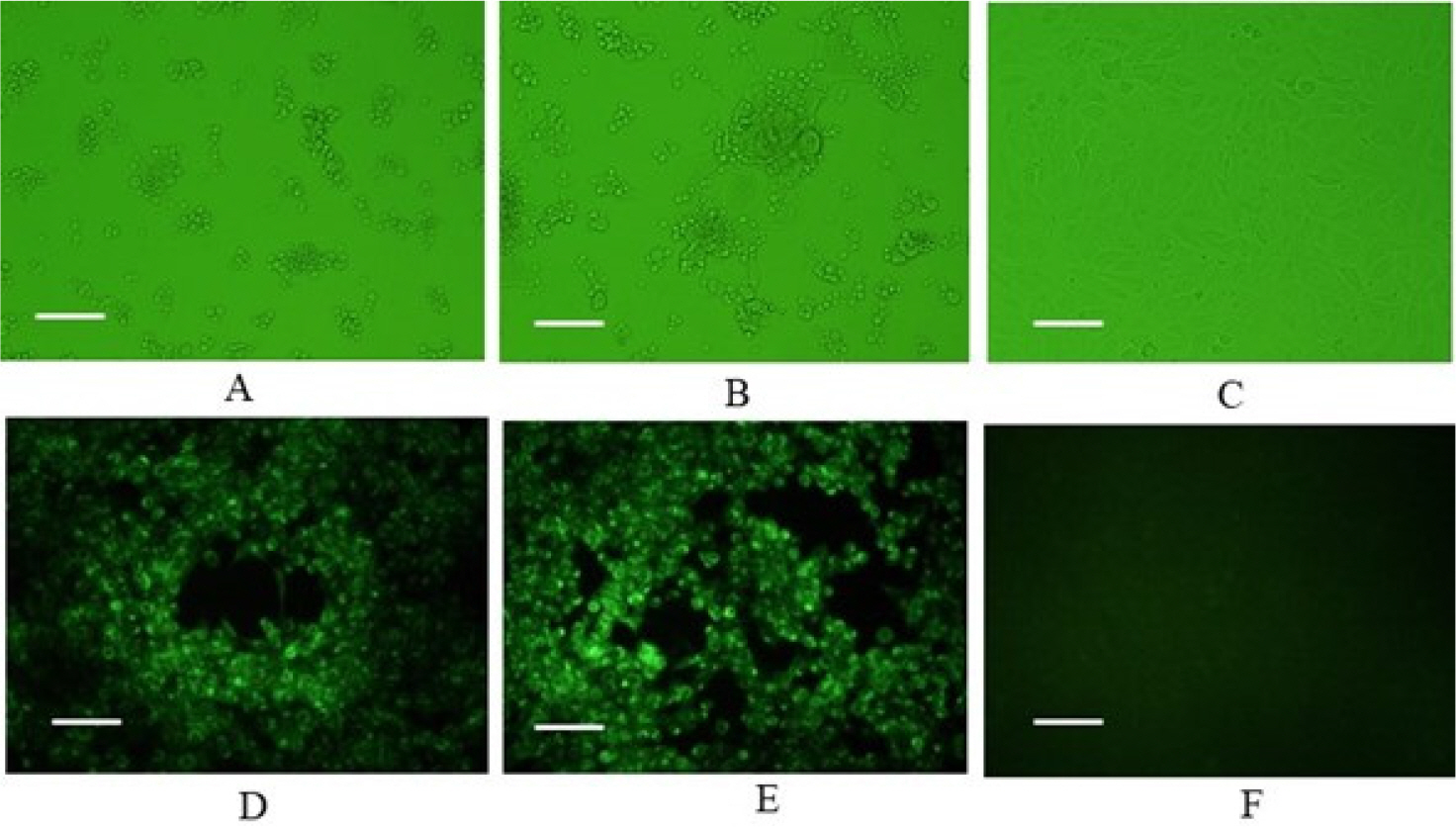
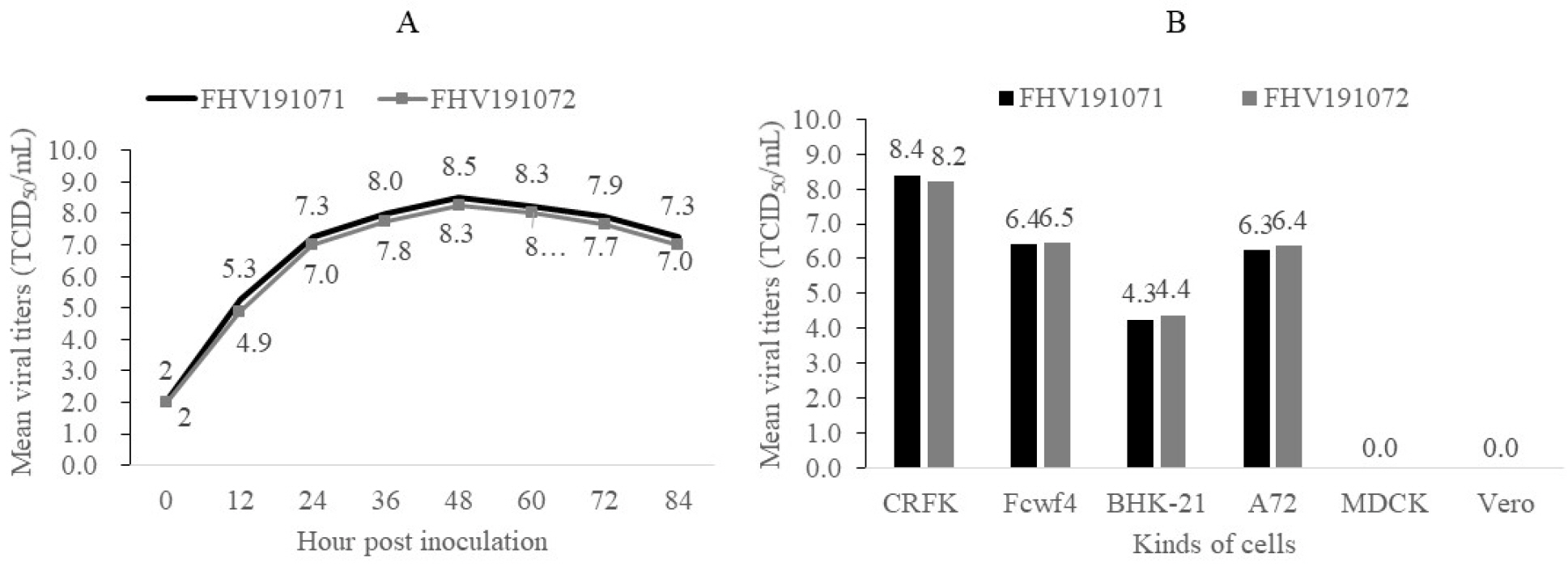
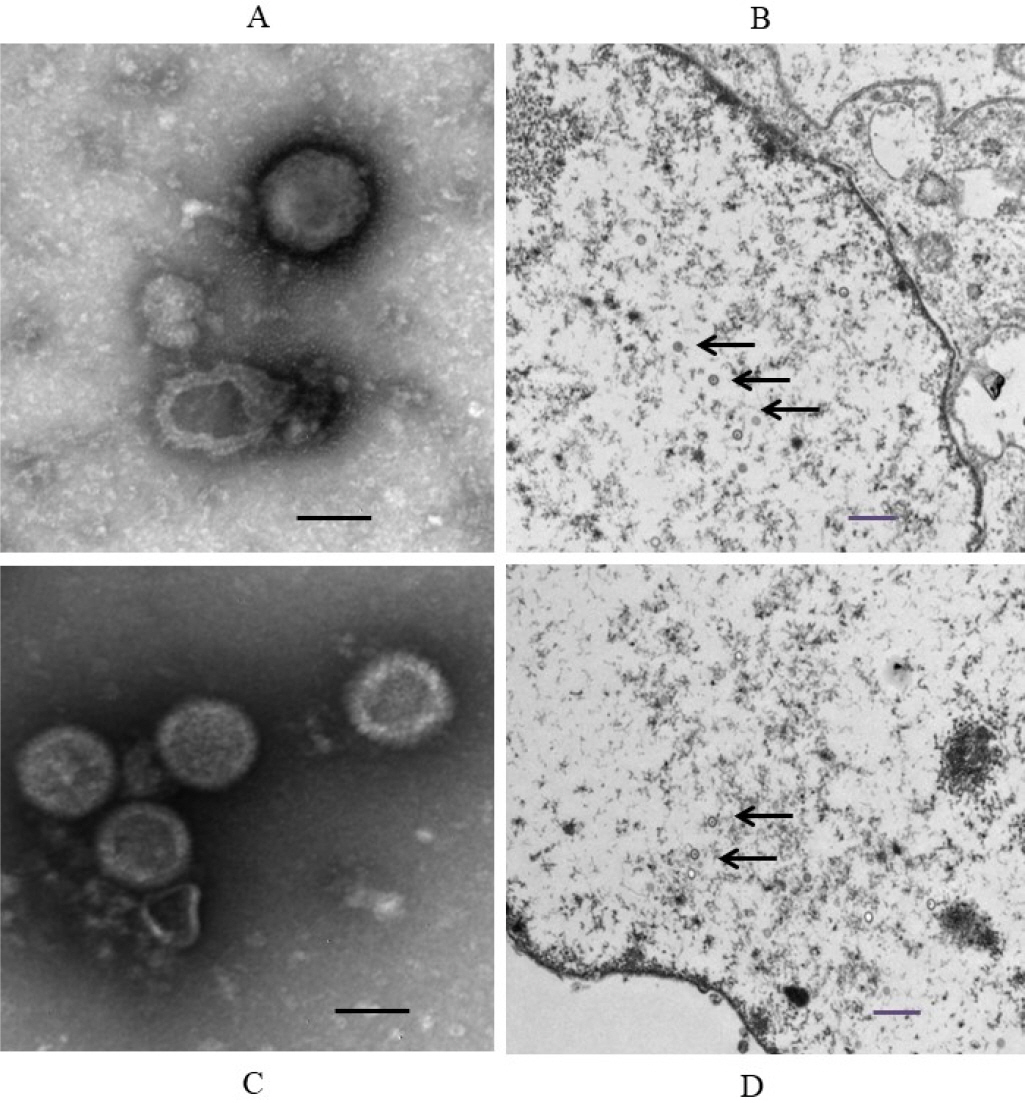
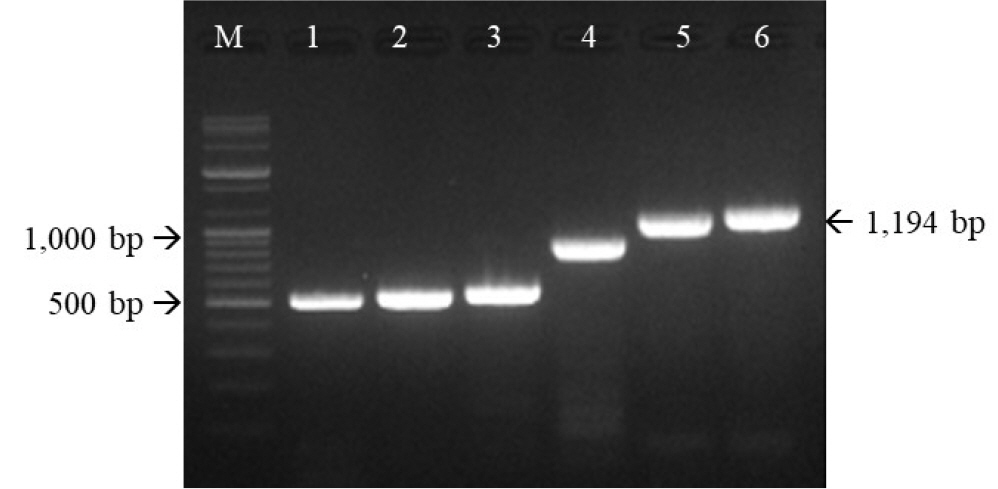
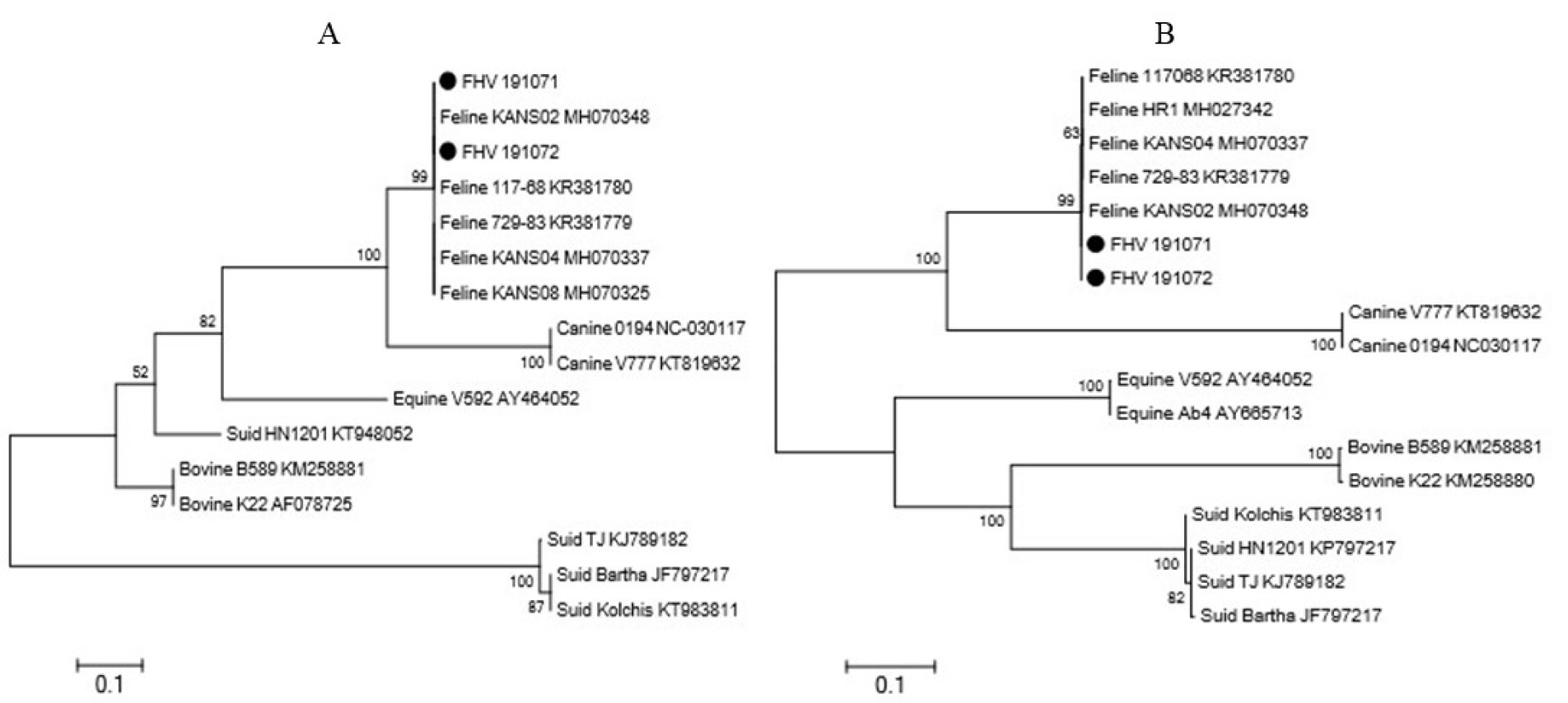
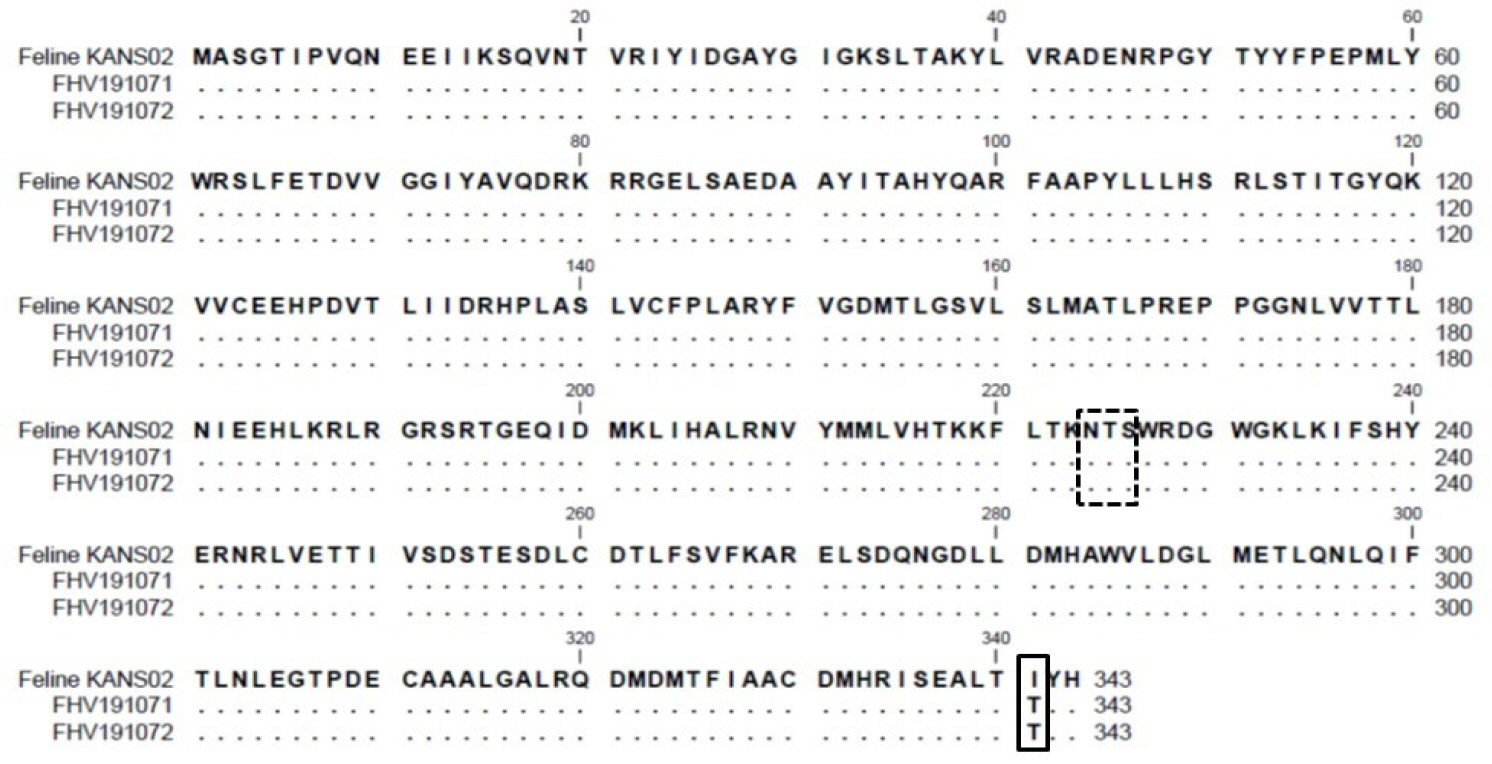
- Feline herpesvirus type 1 (FHV-1) causes respiratory and ocular disease in cats. Although isolates of FHV-1 circulating in cats have been reported worldwide, Korean FHV-1 isolates and their features have not been reported thus far. We aimed to investigate the biological and molecular characterization of two FHV-1 isolates based on the nucleotide sequence of thymidine kinase (TK) and glycoprotein B (gB) gene. In total, 48 samples from 12 cats were prepared for virus isolation.For the diagnosis, virus isolation, indirect fluorescence assay (IFA), electron microscopy (EM), and polymerase chain reaction (PCR) and for the molecular characterization, cloning and sequencing were used. Based on many methods such as virus isolation with specific cytopathic effects, IFA, EM, and PCR, two isolates were confirmed as FHV-1 and they showed the highest viral titer (108.3 to 108.5 TCID50 /mL) in the Crandell–Rees Feline Kidney cells at 48 h after inoculation, but did not grow in MDCK and Vero cells. The nucleotide and amino acid sequences of the full TK and gB gene of FHV191071 and FHV191072 isolates were determined and compared with those of other herpesvirus strains. Two isolates possessed the same nucleotide sequences belonging to FHV-1 group and had the highest similarity (99.9%) with the KANS-02 strain, which was isolated from shelter in USA in 2016. Two isolates were confirmed as FHV-1 and they will be a useful basic resource for evaluating current FHV-1 vaccine and developing diagnostic tools.
Figure
Reference
-
1. Lewin AC, Kolb AW, McLellan GJ, Bentley E, Bernard KA, Newbury SP, et al. Genomic, Recombinational and Phylogenetic Characterization of Global Feline Herpesvirus 1 Isolates. Virology 2018;518:385-97.DOI: 10.1016/j.virol.2018.03.018. PMID: 29605685. PMCID: PMC5935452.2. Tai SHS, Niikura M, Cheng HH, Kruger JM, Wise AG, Maes RK. Complete genomic sequence and an infectious BAC clone of feline herpesvirus-1 (FHV-1). Virology 2010;401:215-27.DOI: 10.1016/j.virol.2010.02.021. PMID: 20304455.3. Gaskell R, Dawson S, Radford A, Thiry E. Feline herpesvirus. Vet Res 2007;38:337-54.DOI: 10.1051/vetres:2006063. PMID: 17296160.4. Tan Y, Dong G, Xu H, Niu J, Lu W, Wang K, et al. Development of a cross-priming isothermal amplification assay based on the glycoprotein B gene for instant and rapid detection of feline herpesvirus type 1. Arch Virol 2020;165:743-7.DOI: 10.1007/s00705-020-04526-5. PMID: 31980939.5. Lee Y, Maes R, Tai SS, Soboll Hussey G. Viral replication and innate immunity of feline herpesvirus-1 virulence-associated genes in feline respiratory epithelial cells. Virus Res 2019;264:56-67.DOI: 10.1016/j.virusres.2019.02.013. PMID: 30796929.6. Harbour DA, Howard PE, Gaskell RM. Isolation of feline calicivirus and feline herpesvirus from domestic cats 1980 to 1989. Vet Rec 1991;128:77-80.DOI: 10.1136/vr.128.4.77. PMID: 1850183.7. Henzel A, Brum MCS, Lautert C, Martins M, Lovato LT, Weiblen R. Isolation and identification of feline calicivirus and feline herpesvirus in Southern Brazil. Braz J Microbiol 2012;43:560-8.DOI: 10.1590/S1517-83822012000200017. PMID: 24031864. PMCID: PMC3768834.8. Hora AS, Tonietti PO, Guerra JM, Leme MC, Pena HFJ, Maiorka PC, et al. Felid herpesvirus 1 as a causative agent of severe nonsuppurative meningoencephalitis in a domestic cat. J Clin Microbiol 2013;51:676-9.DOI: 10.1128/JCM.02462-12. PMID: 23152556. PMCID: PMC3553892.9. Nunberg JH, Wright DK, Cole GE, Petrovskis EA, Post LE, Compton T, et al. Identification of the thymidine kinase gene of feline herpesvirus: use of degenerate oligonucleotides in the polymerase chain reaction to isolate herpesvirus gene homologs. J Virol 1989;63:3240-9.DOI: 10.1128/JVI.63.8.3240-3249.1989. PMID: 2746729. PMCID: PMC250894.10. Gould D. Feline herpesvirus-1: ocular manifestations, diagnosis and treatment options. J Feline Med Surg 2011;13:333-46.DOI: 10.1016/j.jfms.2011.03.010. PMID: 21515221.11. Maes R. Felid herpesvirus type 1 infection in cats: a natural host model for alphaherpesvirus pathogenesis. ISRN Vet Sci 2012;2012:495830.DOI: 10.5402/2012/495830. PMID: 23762586. PMCID: PMC3671728.12. Helps C, Reeves N, Egan K, Howard P, Harbour D. Detection of Chlamydophila felis and feline herpesvirus by multiplex real-time PCR analysis. J Clin Microbiol 2003;41:2734-6.DOI: 10.1128/JCM.41.6.2734-2736.2003. PMID: 12791917. PMCID: PMC156487.13. Litster A, Wu CC, Leutenegger CM. Detection of feline upper respiratory tract disease pathogens using a commercially available real-time PCR test. Vet J 2015;206:149-53.DOI: 10.1016/j.tvjl.2015.08.001. PMID: 26324635.14. Yagami K, Furukawa T, Fukui M. Serologic and virologic surveys on feline herpesvirus and feline calicivirus infections in cats for experimental use. Jikken Dobutsu 1985;34:241-8.DOI: 10.1538/expanim1978.34.3_241. PMID: 2998829.15. Sun H, Li Y, Jiao W, Liu C, Liu X, Wang H, et al. Isolation and identification of feline herpesvirus type 1 from a South China tiger in China. Viruses 2014;6:1004-14.DOI: 10.3390/v6031004. PMID: 24590411. PMCID: PMC3970135.16. Kang BT, Park HM. Prevalence of feline herpesvirus 1, feline calicivirus and Chlamydophila felis in clinically normal cats at a Korean animal shelter. J Vet Sci 2008;9:207-9.DOI: 10.4142/jvs.2008.9.2.207. PMID: 18487944. PMCID: PMC2839100.17. Liu MZ, Han XH, Yao LQ, Zhang WK, Liu BS, Chen ZL. Development and application of a simple recombinase polymerase amplification assay for rapid point-of-care detection of feline herpesvirus type 1. Arch Virol 2019;164:195-200.DOI: 10.1007/s00705-018-4064-7. PMID: 30302584. PMCID: PMC7086775.18. Hoferer M, Braun A, Sting R. Creation of a bovine herpes virus 1 (BoHV-1) quantitative particle standard by transmission electron microscopy and comparison with established standards for use in real-time PCR. Biologicals 2017;48:121-5.DOI: 10.1016/j.biologicals.2017.03.007. PMID: 28456444.19. Abril C, Engels M, Liman A, Hilbe M, Albini S, Franchini M, et al. Both viral and host factors contribute to neurovirulence of bovine herpesviruses 1 and 5 in interferon receptor-deficient mice. J Virol 2004;78:3644-53.DOI: 10.1128/JVI.78.7.3644-3653.2004. PMID: 15016885. PMCID: PMC371052.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prevalence of feline herpesvirus 1, feline calicivirus and Chlamydophila felis in clinically normal cats at a Korean animal shelter
- Successful Treatment of Brugia pahangi in Naturally Infected Cats with Ivermectin
- Immunogenicity of a new inactivated vaccine against feline panleukopenia virus, calicivirus, and herpesvirus-1 for cats
- Incidence and sero-surveillance of feline viruses in Korean cats residing in Gyeonggi-do
- Expression of Toll-like receptors 3, 7, 9 and cytokines in feline infectious peritonitis virus-infected CRFK cells and feline peripheral monocytes