Endocrinol Metab.
2020 Dec;35(4):838-846. 10.3803/EnM.2020.797.
Changes in Glucose Metabolism after Adrenalectomy or Treatment with a Mineralocorticoid Receptor Antagonist for Primary Aldosteronism
- Affiliations
-
- 1Division of Nephrology, Department of Internal Medicine, National Taiwan University Hospital, Taipei, Taiwan
- 2Department of Internal Medicine, National Taiwan University Hospital, Taipei, Taiwan
- 3Division of Endocrinology and Metabolism, Department of Internal Medicine, Taipei Tzu Chi Hospital, The Buddhist Medical Foundation, New Taipei, Taiwan
- 4Division of Urology, Department of Surgery, Far-Eastern Memorial Hospital, New Taipei, Taiwan
- 5Graduate Program in Biomedical Informatics, College of Informatics, Yuan-Ze University, Chung-Li, Taiwan
- KMID: 2511011
- DOI: http://doi.org/10.3803/EnM.2020.797
Abstract
- Background
Data on the effects of excess aldosterone on glucose metabolism are inconsistent. This study compared the changes in glucose metabolism in patients with primary aldosteronism (PA) after adrenalectomy or treatment with a mineralocorticoid receptor antagonist (MRA).
Methods
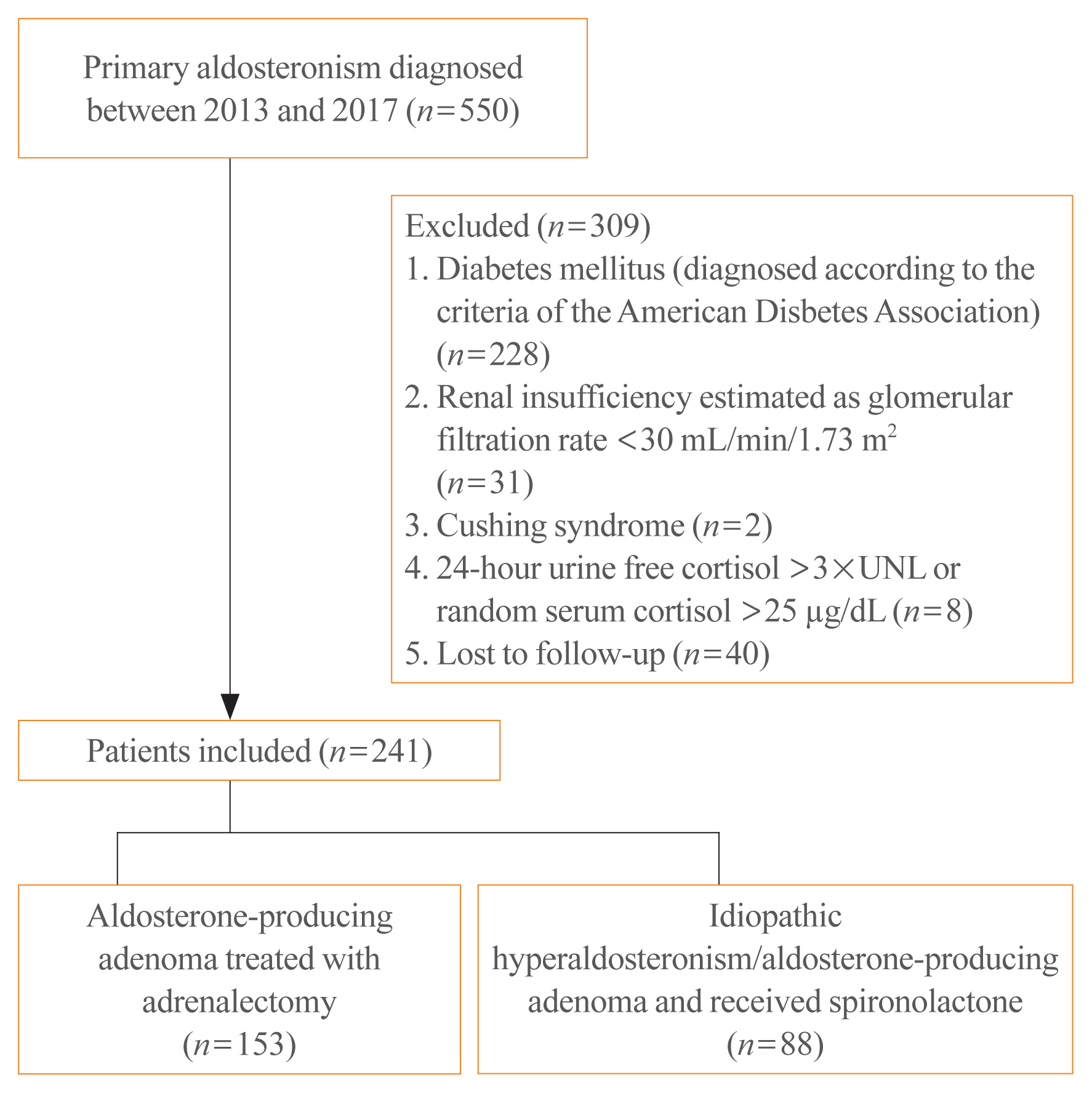
Overall, 241 patients were enrolled; 153 underwent adrenalectomy and 88 received an MRA. Fasting glucose, homeostatic model assessment of insulin resistance (HOMA-IR), and homeostatic model assessment of β-cell function (HOMA-β) were compared between the treatment groups after 1 year. Plasma aldosterone concentration (PAC) and factors determining HOMA-IR and PAC were evaluated.
Results
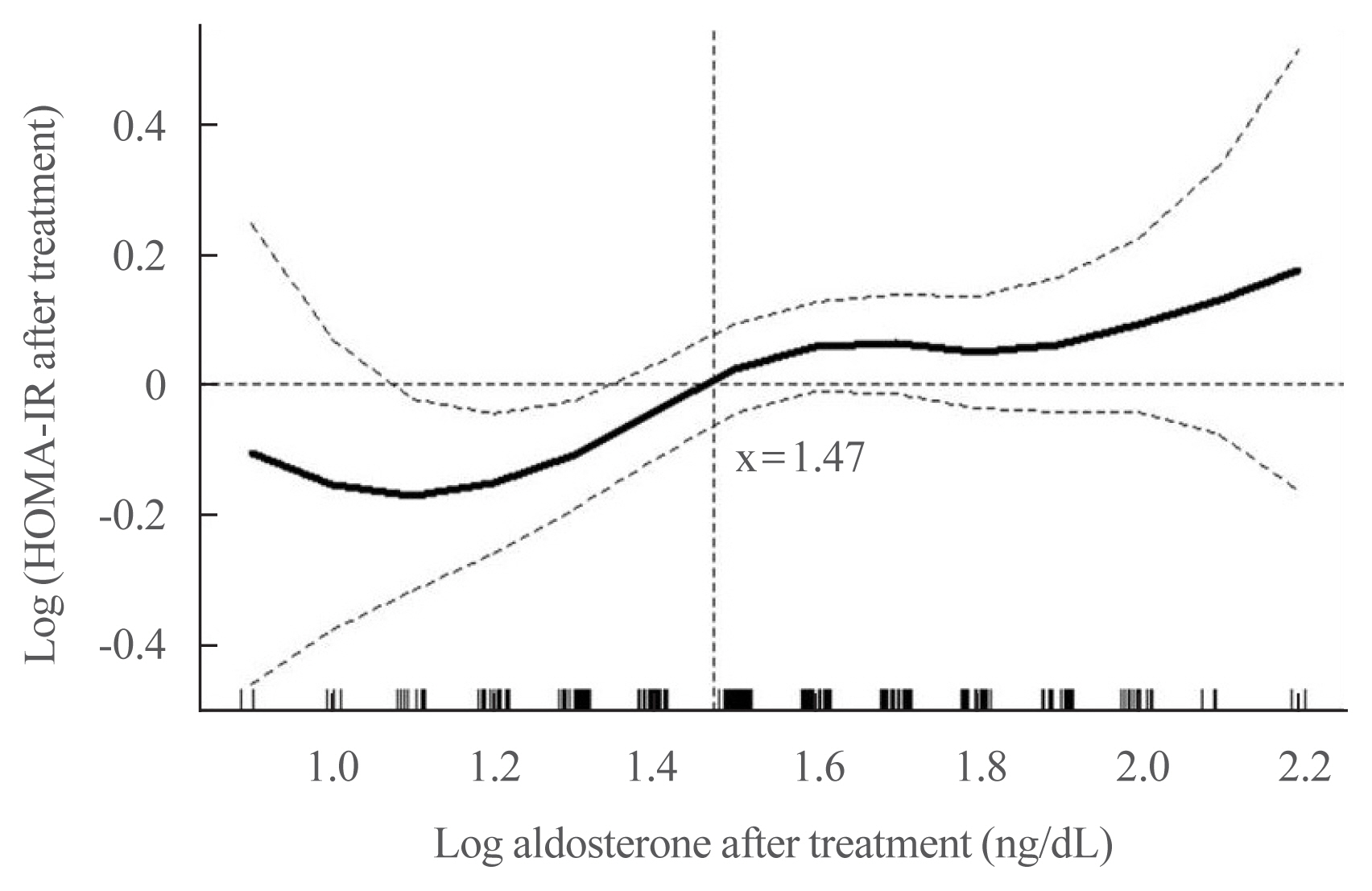
No baseline differences were observed between the groups. Fasting insulin, HOMA-IR, and HOMA-β increased in both groups and there were no significant differences in fasting glucose following treatment. Multiple regression analysis showed associations between PAC and HOMA-IR (β=0.172, P=0.017) after treatment. Treatment with spironolactone was the only risk factor associated with PAC >30 ng/dL (odds ratio, 5.2; 95% confidence interval [CI], 2.7 to 10; P<0.001) and conferred a 2.48-fold risk of insulin resistance after 1 year compared with surgery (95% CI, 1.3 to 4.8; P=0.007).
Conclusion
Spironolactone treatment might increase insulin resistance in patients with PA. This strengthened the current recommendation that adrenalectomy is the preferred strategy for patient with positive lateralization test. Achieving a post-treatment PAC of <30 ng/dL for improved insulin sensitivity may be appropriate.
Figure
Reference
-
1. Conn JW. Hypertension, the potassium ion and impaired carbohydrate tolerance. N Engl J Med. 1965; 273:1135–43.
Article2. Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA. 2015; 314:1021–9.
Article3. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003; 26(Suppl 1):S5–20.4. Kannel WB, Wilson PW, Zhang TJ. The epidemiology of impaired glucose tolerance and hypertension. Am Heart J. 1991; 121(4 Pt 2):1268–73.
Article5. Fallo F, Veglio F, Bertello C, Sonino N, Della Mea P, Ermani M, et al. Prevalence and characteristics of the metabolic syndrome in primary aldosteronism. J Clin Endocrinol Metab. 2006; 91:454–9.
Article6. Ingelsson E, Pencina MJ, Tofler GH, Benjamin EJ, Lanier KJ, Jacques PF, et al. Multimarker approach to evaluate the incidence of the metabolic syndrome and longitudinal changes in metabolic risk factors: the Framingham Offspring Study. Circulation. 2007; 116:984–92.
Article7. Chen W, Li F, He C, Zhu Y, Tan W. Elevated prevalence of abnormal glucose metabolism in patients with primary aldosteronism: a meta-analysis. Ir J Med Sci. 2014; 183:283–91.
Article8. Wu VC, Hu YH, Er LK, Yen RF, Chang CH, Chang YL, et al. Case detection and diagnosis of primary aldosteronism: the consensus of Taiwan Society of Aldosteronism. J Formos Med Assoc. 2017; 116:993–1005.
Article9. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010; 33(Suppl 1):S62–9.10. Ohno Y, Sone M, Inagaki N, Takeda Y, Kurihara I, Tsuiki M, et al. Latent autonomous cortisol secretion from apparently nonfunctioning adrenal tumor in nonlateralized hyperaldosteronism. J Clin Endocrinol Metab. 2019; 104:4382–9.
Article11. Lee CH, Shih AZ, Woo YC, Fong CH, Leung OY, Janus E, et al. Optimal cut-offs of homeostasis model assessment of insulin resistance (HOMA-IR) to identify dysglycemia and type 2 diabetes mellitus: a 15-year prospective study in Chinese. PLoS One. 2016; 11:e0163424.
Article12. Hin LY, Lau TK, Rogers MS, Chang AM. Dichotomization of continuous measurements using generalized additive modelling: application in predicting intrapartum caesarean delivery. Stat Med. 1999; 18:1101–10.13. Ferrannini E. Insulin resistance versus insulin deficiency in non-insulin-dependent diabetes mellitus: problems and prospects. Endocr Rev. 1998; 19:477–90.
Article14. Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest. 1999; 104:787–94.
Article15. Tsurutani Y, Sugisawa C, Ishida A, Inoue K, Saito J, Omura M, et al. Aldosterone excess may inhibit insulin secretion: a comparative study on glucose metabolism pre- and post-adrenalectomy in patients with primary aldosteronism. Endocr J. 2017; 64:339–46.
Article16. Luther JM, Gainer JV, Murphey LJ, Yu C, Vaughan DE, Morrow JD, et al. Angiotensin II induces interleukin-6 in humans through a mineralocorticoid receptor-dependent mechanism. Hypertension. 2006; 48:1050–7.
Article17. Wada T, Ohshima S, Fujisawa E, Koya D, Tsuneki H, Sasaoka T. Aldosterone inhibits insulin-induced glucose uptake by degradation of insulin receptor substrate (IRS) 1 and IRS2 via a reactive oxygen species-mediated pathway in 3T3-L1 adipocytes. Endocrinology. 2009; 150:1662–9.
Article18. Colussi G, Catena C, Lapenna R, Nadalini E, Chiuch A, Sechi LA. Insulin resistance and hyperinsulinemia are related to plasma aldosterone levels in hypertensive patients. Diabetes Care. 2007; 30:2349–54.
Article19. Giacchetti G, Sechi LA, Rilli S, Carey RM. The renin-angiotensin-aldosterone system, glucose metabolism and diabetes. Trends Endocrinol Metab. 2005; 16:120–6.
Article20. Modan M, Halkin H, Almog S, Lusky A, Eshkol A, Shefi M, et al. Hyperinsulinemia: a link between hypertension obesity and glucose intolerance. J Clin Invest. 1985; 75:809–17.
Article21. Ferrannini E, Buzzigoli G, Bonadonna R, Giorico MA, Oleggini M, Graziadei L, et al. Insulin resistance in essential hypertension. N Engl J Med. 1987; 317:350–7.
Article22. Shen DC, Shieh SM, Fuh MM, Wu DA, Chen YD, Reaven GM. Resistance to insulin-stimulated-glucose uptake in patients with hypertension. J Clin Endocrinol Metab. 1988; 66:580–3.
Article23. Reincke M, Meisinger C, Holle R, Quinkler M, Hahner S, Beuschlein F, et al. Is primary aldosteronism associated with diabetes mellitus?: results of the German Conn’s Registry. Horm Metab Res. 2010; 42:435–9.
Article24. Wu VC, Chueh SJ, Chen L, Chang CH, Hu YH, Lin YH, et al. Risk of new-onset diabetes mellitus in primary aldosteronism: a population study over 5 years. J Hypertens. 2017; 35:1698–708.25. Gerards J, Heinrich DA, Adolf C, Meisinger C, Rathmann W, Sturm L, et al. Impaired glucose metabolism in primary aldosteronism is associated with cortisol cosecretion. J Clin Endocrinol Metab. 2019; 104:3192–202.
Article26. Williams TA, Lenders JW, Mulatero P, Burrello J, Rottenkolber M, Adolf C, et al. Outcomes after adrenalectomy for unilateral primary aldosteronism: an international consensus on outcome measures and analysis of remission rates in an international cohort. Lancet Diabetes Endocrinol. 2017; 5:689–99.
Article27. DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979; 237:E214–23.
Article28. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985; 28:412–9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Advances in the management of diabetic kidney disease: beyond sodium-glucose co-transporter 2 inhibitors
- Factors Associated with Resolution of Hypertension after Adrenalectomy in Patients with Primary Aldosteronism
- Anesthetic Experience of Adrenalectomy with Primary Aldosteronism
- Secondary Hypertension Caused by Endocrine Disorders Except Primary Aldosteronism and Pheochromocytoma
- Primary Aldosteronism: An Anesthetic Experience with Adrenalectomy