Cancer Res Treat.
2021 Jan;53(1):77-86. 10.4143/crt.2020.543.
Sequential Treatment with an Immune Checkpoint Inhibitor Followed by a Small-Molecule Targeted Agent Increases Drug-Induced Pneumonitis
- Affiliations
-
- 1Department of Internal Medicine, National Cancer Center, Goyang, Korea
- 2Department of Radiology, National Cancer Center, Goyang, Korea
- 3Department of Pathology, National Cancer Center, Goyang, Korea
- KMID: 2510649
- DOI: http://doi.org/10.4143/crt.2020.543
Abstract
- Purpose
Immune checkpoint inhibitors (ICI) and targeted small-molecule drugs are mainstay elements of lung cancer chemotherapy. However, they are associated with development of pneumonitis, a rare, but potentially life-threatening event. We analyzed lung cancer patients treated with ICI to evaluate the effect of sequential therapeutic administration on the incidence of pneumonitis.
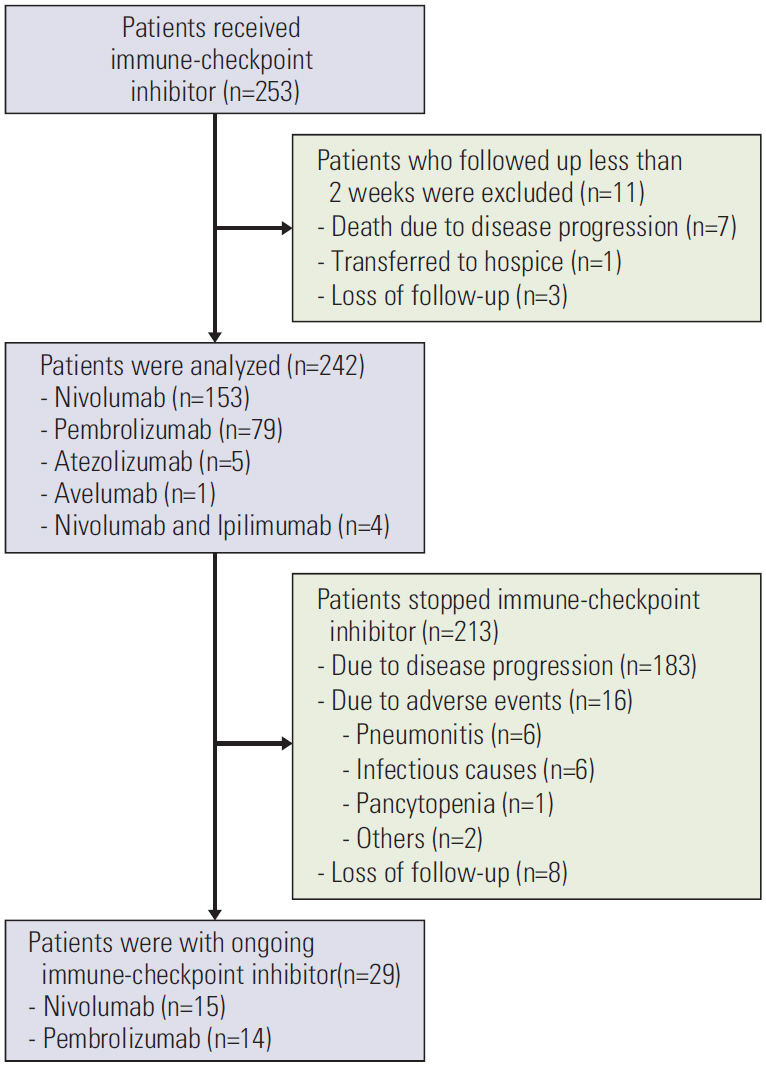
Materials and Methods
In this retrospective study, 242 patients were included. Serial radiologic findings taken during and immediately after ICI treatment were reviewed. Factors that increased pneumonitis and the relationship between peri-ICI chemotherapy and the development of pneumonitis were evaluated.
Results
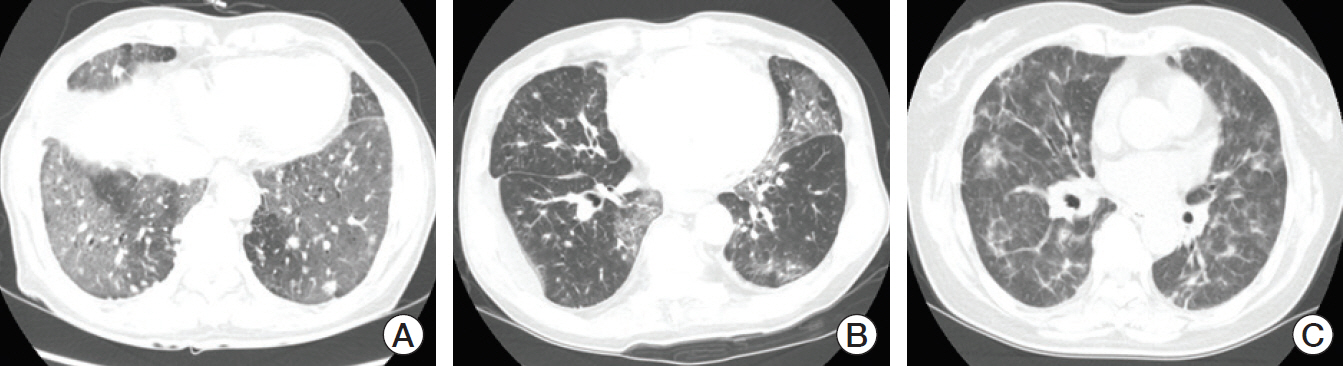
Pneumonitis developed in 23 patients (9.5%); severe pneumonitis (grade ≥ 3) occurred in 13 of 23 patients (56%); pneumonitis-related death occurred in six. High-dose thoracic radiation (≥ 6,000 cGy) revealed a tendency toward high risk of pneumonitis (odds ratio, 2.642; 95% confidence interval, 0.932 to 7.490; p=0.068). Among 149 patients followed for ≥ 8 weeks after the final ICI dose, more patients who received targeted agents within 8-weeks post-ICI experienced pneumonitis (3/16, 18.8%) compared with patients who received cytotoxic agents (4/54, 7.4%) or no chemotherapy (4/79, 5.1%) (p=0.162). Targeted therapy was associated with earlier-onset pneumonitis than treatment with cytotoxic agents (35 vs. 62 days post-ICI, p=0.007); the resulting pneumonitis was more severe (grade ≥ 3, 100% vs. 0%, p=0.031).
Conclusion
Sequential administration of small-molecule targeted agents immediately after ICI may increase the risk of severe pneumonitis. The sequence of chemotherapy regimens that include ICI and targeted agents should be carefully planned to reduce the risk of pneumonitis in lung cancer patients.
Figure
Reference
-
References
1. Lonberg N, Korman AJ. Masterful antibodies: checkpoint blockade. Cancer Immunol Res. 2017; 5:275–81.
Article2. Tolcher AW, Sznol M, Hu-Lieskovan S, Papadopoulos KP, Patnaik A, Rasco DW, et al. Phase Ib study of utomilumab (PF-05082566), a 4-1BB/CD137 agonist, in combination with pembrolizumab (MK-3475) in patients with advanced solid tumors. Clin Cancer Res. 2017; 23:5349–57.
Article3. Azzoli CG, Baker S Jr, Temin S, Pao W, Aliff T, Brahmer J, et al. American Society of Clinical Oncology Clinical Practice Guideline update on chemotherapy for stage IV non-small-cell lung cancer. J Clin Oncol. 2009; 27:6251–66.
Article4. Ardizzoni A, Boni L, Tiseo M, Fossella FV, Schiller JH, Paesmans M, et al. Cisplatin- versus carboplatin-based chemotherapy in first-line treatment of advanced non-small-cell lung cancer: an individual patient data meta-analysis. J Natl Cancer Inst. 2007; 99:847–57.
Article5. Azzoli CG, Kris MG, Pfister DG. Cisplatin versus carboplatin for patients with metastatic non-small-cell lung cancer--an old rivalry renewed. J Natl Cancer Inst. 2007; 99:828–9.
Article6. Shi Y, Au JS, Thongprasert S, Srinivasan S, Tsai CM, Khoa MT, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol. 2014; 9:154–62.
Article7. Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018; 378:113–25.8. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009; 361:947–57.
Article9. Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016; 375:1823–33.
Article10. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015; 373:1627–39.
Article11. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012; 366:2443–54.
Article12. Ma K, Lu Y, Jiang S, Tang J, Li X, Zhang Y. The relative risk and incidence of immune checkpoint inhibitors related pneumonitis in patients with advanced cancer: a meta-analysis. Front Pharmacol. 2018; 9:1430.
Article13. Skeoch S, Weatherley N, Swift AJ, Oldroyd A, Johns C, Hayton C, et al. Drug-induced interstitial lung disease: a systematic review. J Clin Med. 2018; 7:356.
Article14. Cui P, Liu Z, Wang G, Ma J, Qian Y, Zhang F, et al. Risk factors for pneumonitis in patients treated with anti-programmed death-1 therapy: a case-control study. Cancer Med. 2018; 7:4115–20.
Article15. Nishino M, Giobbie-Hurder A, Hatabu H, Ramaiya NH, Hodi FS. Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: a systematic review and meta-analysis. JAMA Oncol. 2016; 2:1607–16.16. Gemma A, Kusumoto M, Kurihara Y, Masuda N, Banno S, Endo Y, et al. Interstitial lung disease onset and its risk factors in Japanese patients with ALK-positive NSCLC after treatment with crizotinib. J Thorac Oncol. 2019; 14:672–82.
Article17. Oshima Y, Tanimoto T, Yuji K, Tojo A. EGFR-TKI-associated interstitial pneumonitis in nivolumab-treated patients with non-small cell lung cancer. JAMA Oncol. 2018; 4:1112–5.
Article18. Lim ZF, Ma PC. Emerging insights of tumor heterogeneity and drug resistance mechanisms in lung cancer targeted therapy. J Hematol Oncol. 2019; 12:134.
Article19. Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010; 28:3167–75.
Article20. Schoenfeld AJ, Arbour KC, Rizvi H, Iqbal AN, Gadgeel SM, Girshman J, et al. Severe immune-related adverse events are common with sequential PD-(L)1 blockade and osimertinib. Ann Oncol. 2019; 30:839–44.
Article21. Moya-Horno I, Viteri S, Karachaliou N, Rosell R. Combination of immunotherapy with targeted therapies in advanced non-small cell lung cancer (NSCLC). Ther Adv Med Oncol. 2018; 10:1758834017745012.
Article22. Lin JJ, Chin E, Yeap BY, Ferris LA, Kamesan V, Lennes IT, et al. Increased hepatotoxicity associated with sequential immune checkpoint inhibitor and crizotinib therapy in patients with non-small cell lung cancer. J Thorac Oncol. 2019; 14:135–40.
Article23. Longoria TC, Tewari KS. Evaluation of the pharmacokinetics and metabolism of pembrolizumab in the treatment of melanoma. Expert Opin Drug Metab Toxicol. 2016; 12:1247–53.
Article24. Mizoguchi K, Nakamura Y, Sano K, Sato S, Ikegami Y, Motoshima K, et al. Pharmacokinetic parameters of gefitinib predict efficacy and toxicity in patients with advanced non-small cell lung cancer harboring EGFR mutations. Cancer Chemother Pharmacol. 2016; 78:377–82.
Article25. Palma DA, Senan S, Tsujino K, Barriger RB, Rengan R, Moreno M, et al. Predicting radiation pneumonitis after chemoradiation therapy for lung cancer: an international individual patient data meta-analysis. Int J Radiat Oncol Biol Phys. 2013; 85:444–50.
Article26. Nakahama K, Tamiya A, Isa SI, Taniguchi Y, Shiroyama T, Suzuki H, et al. Association between imaging findings of airway obstruction adjacent to lung tumors and the onset of interstitial lung disease after nivolumab. In Vivo. 2018; 32:887–91.
Article27. Katsui K, Ogata T, Watanabe K, Katayama N, Soh J, Kuroda M, et al. Dose-volume parameters predict radiation pneumonitis after induction chemoradiotherapy followed by surgery for non-small cell lung cancer: a retrospective analysis. BMC Cancer. 2019; 19:1144.
Article28. Rodrigues G, Choy H, Bradley J, Rosenzweig KE, Bogart J, Curran WJ Jr, et al. Definitive radiation therapy in locally advanced non-small cell lung cancer: executive summary of an American Society for Radiation Oncology (ASTRO) evidence-based clinical practice guideline. Pract Radiat Oncol. 2015; 5:141–8.
Article29. Fukihara J, Sakamoto K, Koyama J, Ito T, Iwano S, Morise M, et al. Prognostic impact and risk factors of immune-related pneumonitis in patients with non-small-cell lung cancer who received programmed death 1 inhibitors. Clin Lung Cancer. 2019; 20:442–50.
Article30. Shinno Y, Goto Y, Ohuchi M, Hamada A, Nokihara H, Fujiwara Y, et al. The long half-life of programmed cell death protein 1 inhibitors may increase the frequency of immune-related adverse events after subsequent EGFR tyrosine kinase inhibitor therapy. JTO Clin Res Rep. 2020; 1:100008.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Rituximab Treatment for Polyneuropathy Induced by an Immune Checkpoint Inhibitor
- Current status of cancer immunotherapy with immune checkpoint inhibitors
- Gut microbiome on immune checkpoint inhibitor therapy and consequent immune-related colitis: a review
- Treatment of advanced urogenital cancers with immune checkpoint inhibitors
- Breakthroughs in the Systemic Treatment of HER2-Positive Advanced/Metastatic Gastric Cancer: From Singlet Chemotherapy to Triple Combination