Blood Res.
2020 Dec;55(4):217-224. 10.5045/br.2020.2020127.
Outcomes of pediatric acute myeloid leukemia patients with FLT3-ITD mutations in the pre-FLT3 inhibitor era
- Affiliations
-
- 1Department of Pediatrics, Seoul National University Children’s Hospital, Seoul National University College of Medicine, Seoul, Korea
- 2Seoul National University Cancer Research Institute, Seoul, Korea
- 3Wide River Institute of Immunology, Hongcheon, Korea
- KMID: 2509961
- DOI: http://doi.org/10.5045/br.2020.2020127
Abstract
- Background
Acute myeloid leukemia (AML) with internal tandem duplication in FMS-like tyrosine kinase 3 (FLT3-ITD) is associated with poor outcomes. This study aimed to analyze the outcomes of pediatric AML patients with FLT3-ITD mutations in the pre-FLT3 inhibitor era.
Methods
We retrospectively reviewed and identified 18 patients diagnosed with non-M3 AML with FLT3-ITD mutations at Seoul National University Children’s Hospital between May 2008 and August 2019.
Results
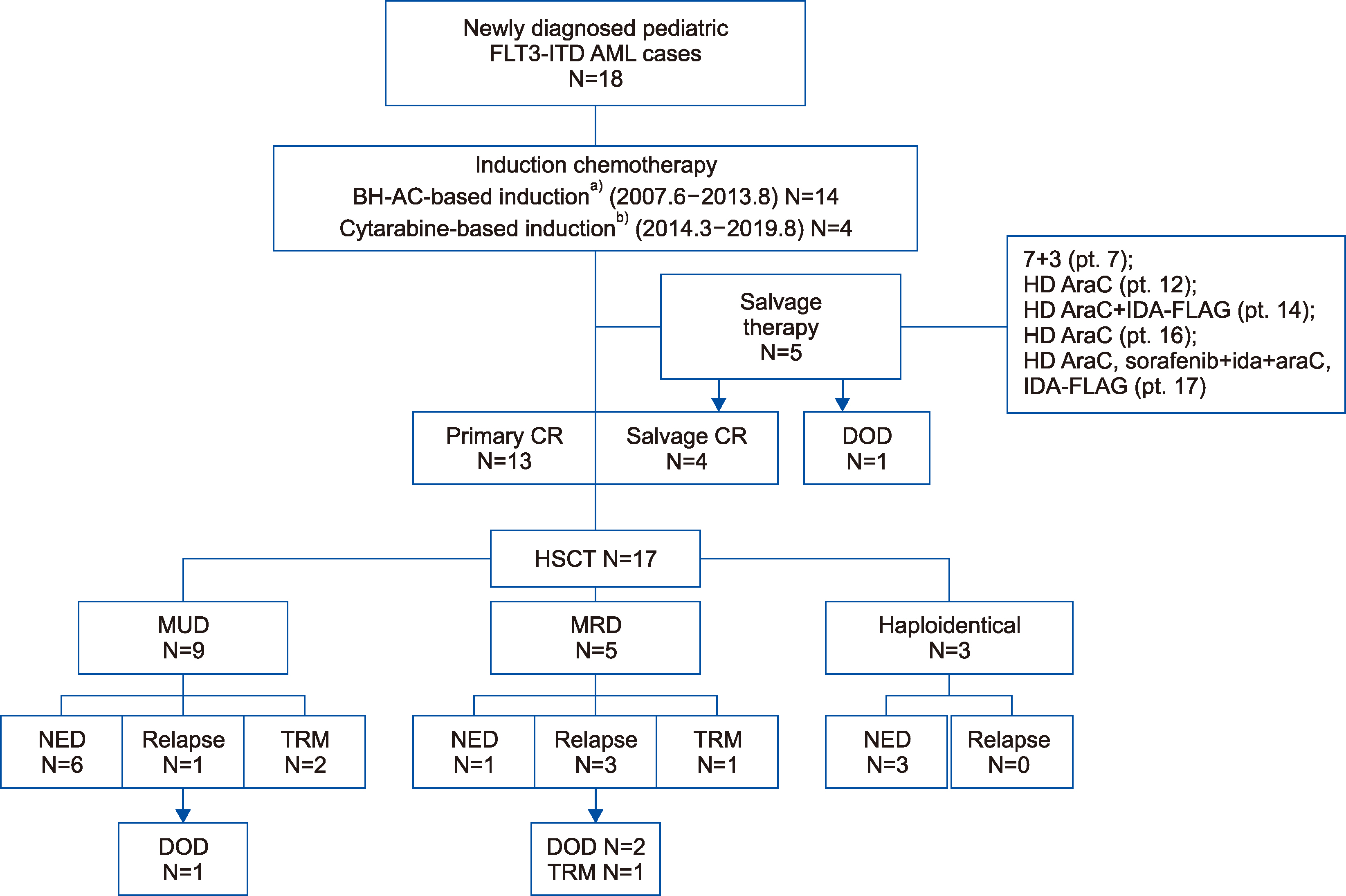
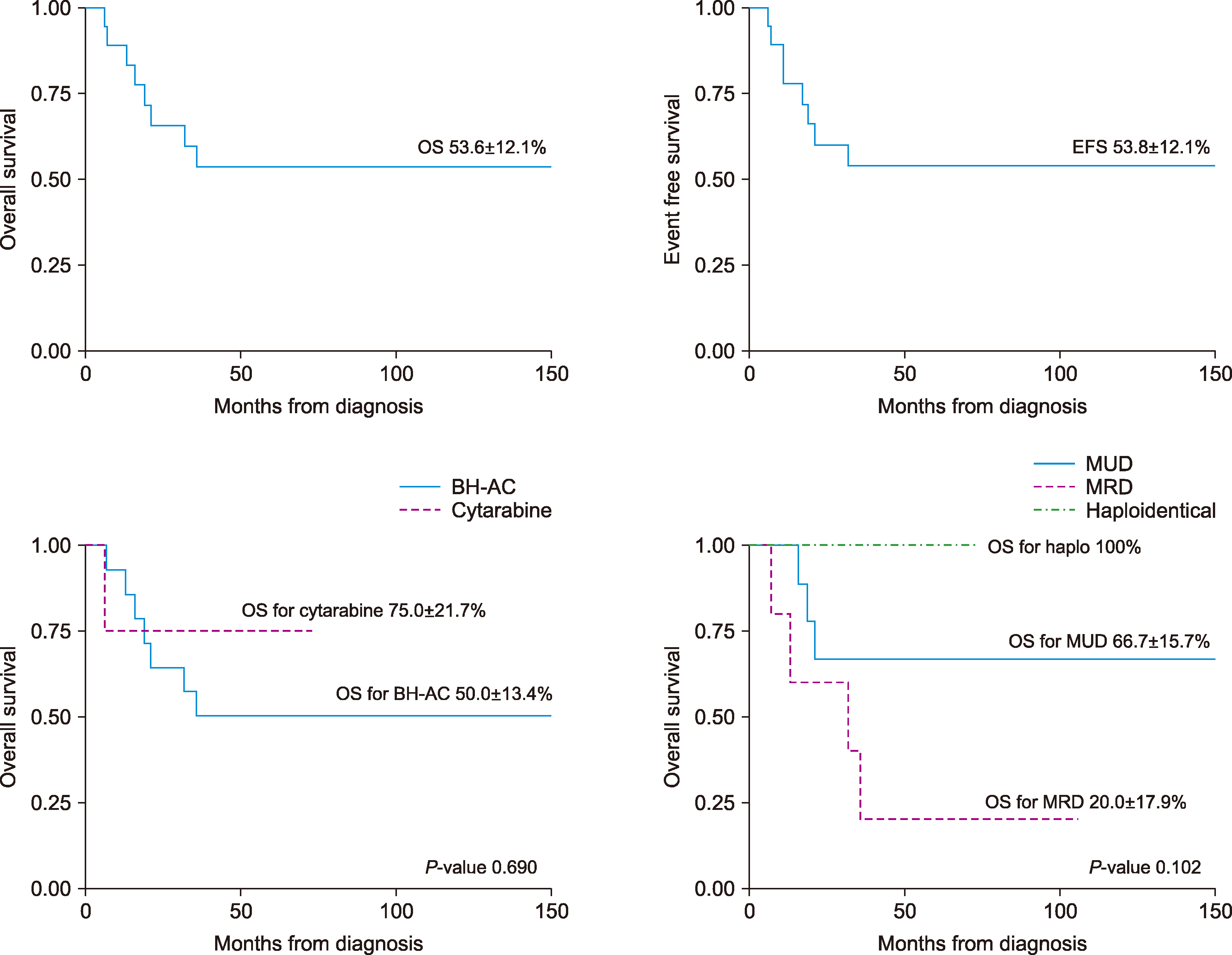
The median age was 13 years (range, 6‒19 yr). The median follow-up time was 43 months (range, 6‒157 mo). Fourteen patients received BH-AC-based (N4-Behenoy1-1-β-D-arabinofuranosy1cytosine) and 4 received cytarabine-based induction chemotherapy. Complete remission (CR) was achieved in 72.2% of the patients after the first induction chemotherapy and 80% of the patients achieved CR after salvage therapy. The overall CR rate was 94% (17/18 patients). These 17 patients underwent hematopoietic stem cell transplantation (9 matched unrelated donors, 5 matched related donors, and 3 haploidentical donors). Relapse occurred in 22% of the patients. Event free survival and overall survival rates were 53.8±12.1% and 53.6±12.1%, respectively, and they were not significantly different according to the type of induction chemotherapy (P=0.690) or the type of donor (P =0.102).
Conclusion
This study outlines the outcomes of pediatric AML patients with FLT3-ITD-mutations in one institution over a decade. Outcomes were significantly improved in this study compared to our previous report in 2004, where RFS and EFS were 0%. This study can provide baseline data for pediatric patients in the pre-FLT3 inhibitor era.
Keyword
Figure
Reference
-
1. Qiu QC, Wang C, Bao XB, et al. 2018; The impact of FLT3 mutations on treatment response and survival in Chinese de novo AML patients. Hematology. 23:131–8. DOI: 10.1080/10245332.2017.1372248. PMID: 28876197.2. Smith CC, Wang Q, Chin CS, et al. 2012; Validation of ITD mutations in FLT3 as a therapeutic target in human acute myeloid leukaemia. Nature. 485:260–3. DOI: 10.1038/nature11016. PMID: 22504184. PMCID: PMC3390926.
Article3. Song TY, Lee SH, Kim G, Baek HJ, Hwang TJ, Kook H. 2018; Improvement of treatment outcome over 2 decades in children with acute myeloid leukemia. Blood Res. 53:25–34. DOI: 10.5045/br.2018.53.1.25. PMID: 29662859. PMCID: PMC5898990.
Article4. Fenski R, Flesch K, Serve S, et al. 2000; Constitutive activation of FLT3 in acute myeloid leukaemia and its consequences for growth of 32D cells. Br J Haematol. 108:322–30. DOI: 10.1046/j.1365-2141.2000.01831.x. PMID: 10691863.
Article5. Badar T, Kantarjian HM, Nogueras-Gonzalez GM, et al. 2015; Improvement in clinical outcome of FLT3 ITD mutated acute myeloid leukemia patients over the last one and a half decade. Am J Hematol. 90:1065–70. DOI: 10.1002/ajh.24140. PMID: 26299958. PMCID: PMC4618182.6. Kang HJ, Hong SH, Kim IH, et al. 2005; Prognostic significance of FLT3 mutations in pediatric non-promyelocytic acute myeloid leukemia. Leuk Res. 29:617–23. DOI: 10.1016/j.leukres.2004.11.006. PMID: 15863200.
Article7. Wu X, Feng X, Zhao X, et al. 2016; Prognostic significance of FLT3-ITD in pediatric acute myeloid leukemia: a meta-analysis of cohort studies. Mol Cell Biochem. 420:121–8. DOI: 10.1007/s11010-016-2775-1. PMID: 27435859.
Article8. Shimada A, Iijima-Yamashita Y, Tawa A, et al. 2018; Risk-stratified therapy for children with FLT3-ITD-positive acute myeloid leukemia: results from the JPLSG AML-05 study. Int J Hematol. 107:586–95. DOI: 10.1007/s12185-017-2395-x. PMID: 29330746.
Article9. Lee JW, Kang HJ, Kim S, et al. 2015; Favorable outcome of hematopoietic stem cell transplantation using a targeted once-daily intravenous busulfan-fludarabine-etoposide regimen in pediatric and infant acute lymphoblastic leukemia patients. Biol Blood Marrow Transplant. 21:190–5. DOI: 10.1016/j.bbmt.2014.09.013. PMID: 25255163.10. Rhee SJ, Lee JW, Yu KS, et al. 2017; Pediatric patients undergoing hematopoietic stem cell transplantation can greatly benefit from a novel once-daily intravenous busulfan dosing nomogram. Am J Hematol. 92:607–13. DOI: 10.1002/ajh.24734. PMID: 28370238.
Article11. Hong KT, Kang HJ, Choi JY, et al. 2018; Favorable outcome of post-transplantation cyclophosphamide haploidentical peripheral blood stem cell transplantation with targeted busulfan-based myeloablative conditioning using intensive pharmacokinetic monitoring in pediatric patients. Biol Blood Marrow Transplant. 24:2239–44. DOI: 10.1016/j.bbmt.2018.06.034. PMID: 29981849.
Article12. Baron F, Labopin M, Ruggeri A, et al. 2018; Impact of donor type in patients with aml given allogeneic hematopoietic cell transplantation after low-dose TBI-based regimen. Clin Cancer Res. 24:2794–803. DOI: 10.1158/1078-0432.CCR-17-3622. PMID: 29555662.
Article13. Versluis J, Labopin M, Ruggeri A, et al. 2017; Alternative donors for allogeneic hematopoietic stem cell transplantation in poor-risk AML in CR1. Blood Adv. 1:477–85. DOI: 10.1182/bloodadvances.2016002386. PMID: 29296964. PMCID: PMC5738980.
Article14. Taylor E, Morris K, Ellis M, et al. 2018; FLT3-ITD positive acute myeloid leukemia: a retrospective analysis of the role of allogeneic transplant and allelic ratio in patient management. Asia Pac J Clin Oncol. 14:426–30. DOI: 10.1111/ajco.12827. PMID: 29383835.
Article15. Linch DC, Hills RK, Burnett AK, Khwaja A, Gale RE. 2014; Impact of FLT3(ITD) mutant allele level on relapse risk in intermediate-risk acute myeloid leukemia. Blood. 124:273–6. DOI: 10.1182/blood-2014-02-554667. PMID: 24855211.
Article16. Schlenk RF, Kayser S, Bullinger L, et al. 2014; Differential impact of allelic ratio and insertion site in FLT3-ITD-positive AML with respect to allogeneic transplantation. Blood. 124:3441–9. DOI: 10.1182/blood-2014-05-578070. PMID: 25270908.
Article17. Kim Y, Lee GD, Park J, et al. 2015; Quantitative fragment analysis of FLT3-ITD efficiently identifying poor prognostic group with high mutant allele burden or long ITD length. Blood Cancer J. 5:e336. DOI: 10.1038/bcj.2015.61. PMID: 26832846. PMCID: PMC4558586.
Article18. Chen F, Sun J, Yin C, et al. 2020; Impact of FLT3-ITD allele ratio and ITD length on therapeutic outcome in cytogenetically normal AML patients without NPM1 mutation. Bone Marrow Transplant. 55:740–8. DOI: 10.1038/s41409-019-0721-z. PMID: 31645666.
Article19. Tao S, Wang C, Chen Y, et al. 2019; Prognosis and outcome of patients with acute myeloid leukemia based on FLT3-ITD mutation with or without additional abnormal cytogenetics. Oncol Lett. 18:6766–74. DOI: 10.3892/ol.2019.11051. PMID: 31807186. PMCID: PMC6876342.
Article20. Daver N, Schlenk RF, Russell NH, Levis MJ. 2019; Targeting FLT3 mutations in AML: review of current knowledge and evidence. Leukemia. 33:299–312. DOI: 10.1038/s41375-018-0357-9. PMID: 30651634. PMCID: PMC6365380.
Article21. Pasic I, Da'na W, Lam W, et al. 2019; Influence of FLT3-ITD and NPM1 status on allogeneic hematopoietic cell transplant outcomes in patients with cytogenetically normal AML. Eur J Haematol. 102:368–74. DOI: 10.1111/ejh.13216. PMID: 30706524.
Article22. Yee KW, Schittenhelm M, O'Farrell AM, et al. 2004; Synergistic effect of SU11248 with cytarabine or daunorubicin on FLT3 ITD-positive leukemic cells. Blood. 104:4202–9. DOI: 10.1182/blood-2003-10-3381. PMID: 15304385.
Article23. Levis M, Pham R, Smith BD, Small D. 2004; In vitro studies of a FLT3 inhibitor combined with chemotherapy: sequence of administration is important to achieve synergistic cytotoxic effects. Blood. 104:1145–50. DOI: 10.1182/blood-2004-01-0388. PMID: 15126317.
Article24. Ravandi F, Kantarjian H, Faderl S, et al. 2010; Outcome of patients with FLT3-mutated acute myeloid leukemia in first relapse. Leuk Res. 34:752–6. DOI: 10.1016/j.leukres.2009.10.001. PMID: 19878996. PMCID: PMC4082769.
Article25. Chappell G, Geer M, Gatza E, et al. 2019; Maintenance sorafenib in FLT3-ITD AML following allogeneic HCT favorably impacts relapse and overall survival. Bone Marrow Transplant. 54:1518–20. DOI: 10.1038/s41409-019-0493-5. PMID: 30809038.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- FLT3 mutations in acute myeloid leukemia: a review focusing on clinically applicable drugs
- Gilteritinib Reduces FLT3 Expression in Acute Myeloid Leukemia Cells
- Prognostic significance of nucleophosmin mutations and FLT3 internal tandem duplication in adult patients with cytogenetically normal acute myeloid leukemia
- Prognostic Significance of FLT3 Internal Tandem Duplication in Acute Myeloid Leukemia with Normal Karyotype
- High Transcript Level of FLT3 Associated with High Risk of Relapse in Pediatric Acute Myeloid Leukemia