Anat Cell Biol.
2020 Dec;53(4):471-480. 10.5115/acb.20.277.
Nicotinamide adenine dinucleotide phosphate oxidase inhibitor induces apoptosis on EpsteinBarr virus positive B lymphoma cells
- Affiliations
-
- 1Department of Internal Medicine, Dongguk University Gyeongju Hospital, Gyeongju, Korea
- 2Division of Pulmonary, Allergy and Critical Care Medicine, Department of Internal Medicine, Inje University Busan Paik Hospital, Busan, Korea
- 3Department of Anatomy and Tumor Immunology, Inje University College of Medicine, Busan, Korea
- KMID: 2509693
- DOI: http://doi.org/10.5115/acb.20.277
Abstract
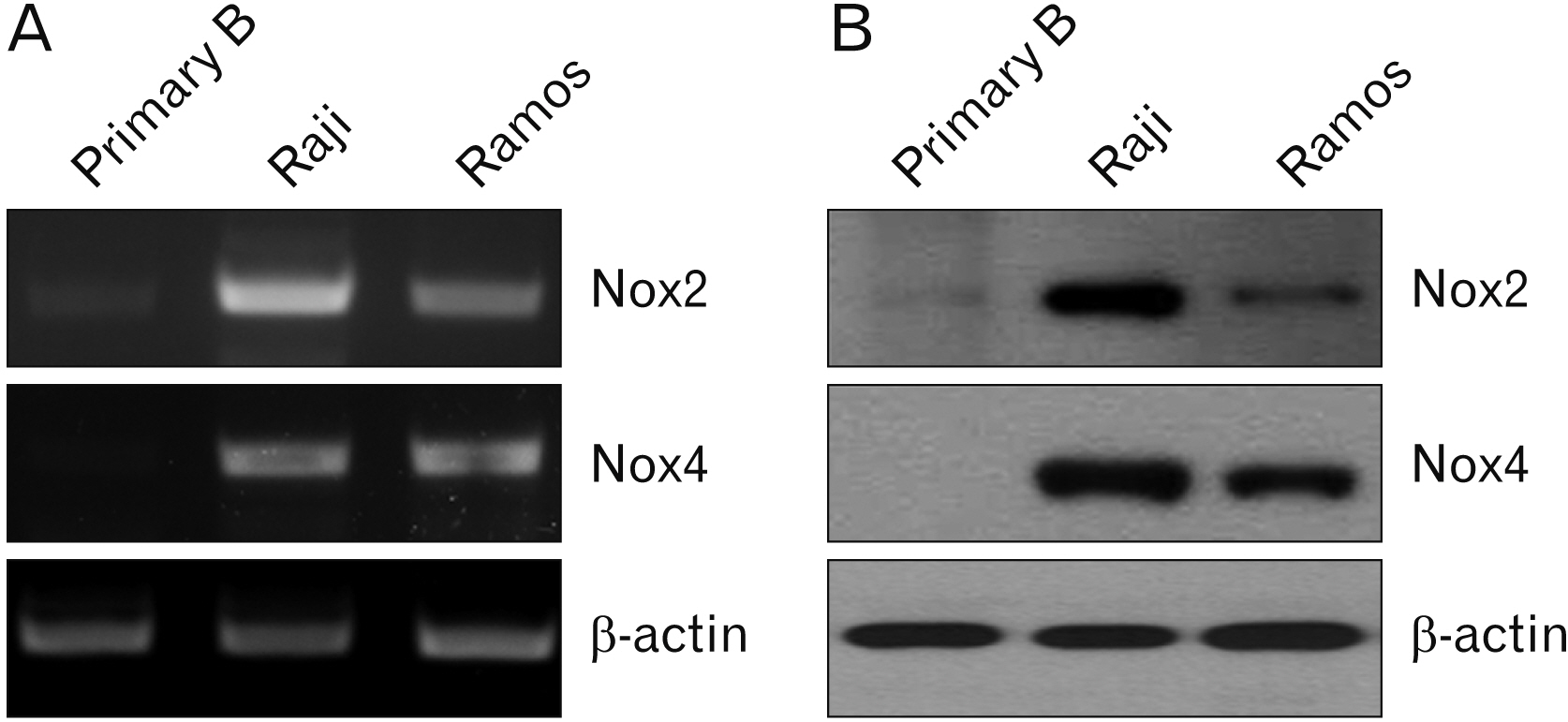
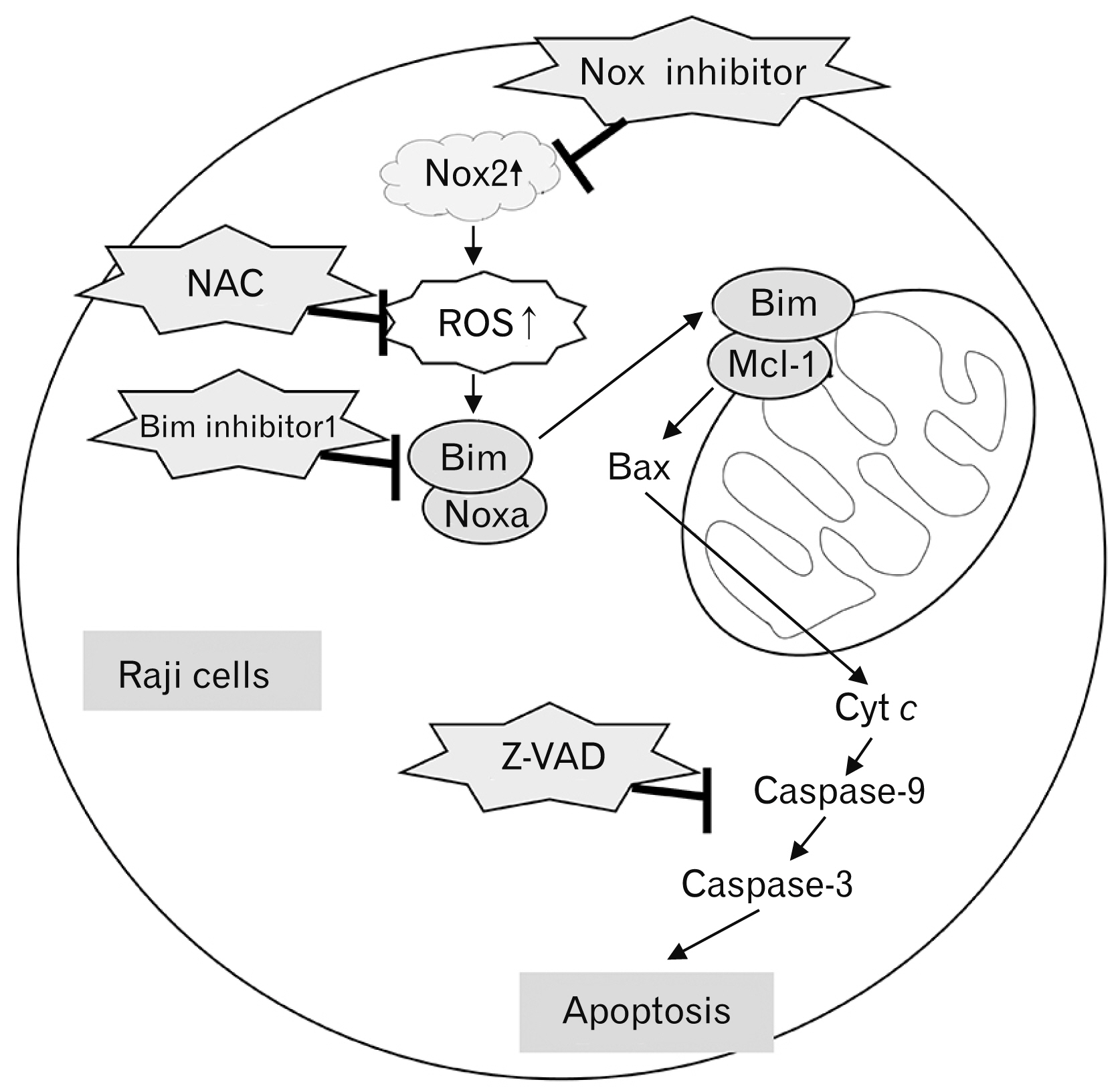
- Over-expression of nicotinamide adenine dinucleotide phosphate oxidase (Nox) isoform enzymes was recently reported in various cancers including Burkitt’s lymphoma (BL). However, the functions of Nox isoform enzymes in BL remain poorly understood. In this study, Nox isoform expression and the effects of a Nox-specific inhibitor were evaluated in Epstein-Barr virus (EBV)-positive Raji BL cells in comparison with EBV-negative Ramos BL cells. To evaluate Nox enzyme expression in Raji and Ramos BL cells, polymerase chain reaction (PCR) and western blot analysis were performed. To verify the intracellular signaling mechanism of the Nox inhibitor-induced apoptosis of Raji cells, WST-1 assay, trypan blue exclusion method, flow cytometry, PCR, western blotting, and bromodeoxyuridine staining were conducted. Experiments using the pan-caspase inhibitor z-VAD, reactive oxygen species scavenger N-acetyl-L-cysteine (NAC), and Bim inhibitor 1 were performed. PCR and western blot results showed that Nox isoform enzymes were highly expressed in EBV-positive BL Raji cells compared with EBV-negative BL Ramos cells. The Nox2 inhibitor induced apoptosis of Raji cells in time- and dosedependent manners. The Nox2 inhibitor also caused up-regulation of Bim and Noxa, down-regulation of Mcl-1, translocation of Bax, release of cytochrome c, and caspase cascade activation, resulting in apoptosis. Furthermore, z-VAD, NAC, and BI-1 effectively blocked the Nox2 inhibitor-induced apoptosis of Raji cells. Taken together, these results provide a novel insight into the mechanism of Nox inhibitor-induced apoptosis and evidence for Nox as a therapeutic target to treat EBV-positive malignancies.
Keyword
Figure
Reference
-
References
1. Kalisz K, Alessandrino F, Beck R, Smith D, Kikano E, Ramaiya NH, Tirumani SH. 2019; An update on Burkitt lymphoma: a review of pathogenesis and multimodality imaging assessment of disease presentation, treatment response, and recurrence. Insights Imaging. 10:56. DOI: 10.1186/s13244-019-0733-7. PMID: 31115699. PMCID: PMC6529494.
Article2. Lu J, Tan H, Li B, Chen S, Xu L, Zou Y. 2020; Status and prognostic nomogram of patients with Burkitt lymphoma. Oncol Lett. 19:972–84. DOI: 10.3892/ol.2019.11155. PMID: 31897210. PMCID: PMC6924199.
Article3. Morton LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS. 2006; Lymphoma incidence patterns by WHO subtype in the United States, 1992-2001. Blood. 107:265–76. DOI: 10.1182/blood-2005-06-2508. PMID: 16150940. PMCID: PMC1895348.
Article4. Ferry JA. 2006; Burkitt's lymphoma: clinicopathologic features and differential diagnosis. Oncologist. 11:375–83. DOI: 10.1634/theoncologist.11-4-375. PMID: 16614233.
Article5. van Vuren A, Meyer-Wentrup F. 2014; New targets for antibody therapy of pediatric B cell lymphomas. Pediatr Blood Cancer. 61:2158–63. DOI: 10.1002/pbc.25193. PMID: 25154500.
Article6. Sandlund JT. 2015; Non-Hodgkin lymphoma in children. Curr Hematol Malig Rep. 10:237–43. DOI: 10.1007/s11899-015-0277-y. PMID: 26174528.
Article7. Dunleavy K. 2018; Approach to the diagnosis and treatment of adult Burkitt's lymphoma. J Oncol Pract. 14:665–71. DOI: 10.1200/JOP.18.00148. PMID: 30423267.
Article8. Casulo C, Friedberg J. 2015; Treating Burkitt lymphoma in adults. Curr Hematol Malig Rep. 10:266–71. DOI: 10.1007/s11899-015-0263-4. PMID: 26013028.
Article9. Molyneux EM, Rochford R, Griffin B, Newton R, Jackson G, Menon G, Harrison CJ, Israels T, Bailey S. 2012; Burkitt's lymphoma. Lancet. 379:1234–44. DOI: 10.1016/S0140-6736(11)61177-X. PMID: 2558562.
Article10. Karpova MB, Schoumans J, Ernberg I, Henter JI, Nordenskjöld M, Fadeel B. 2005; Raji revisited: cytogenetics of the original Burkitt's lymphoma cell line. Leukemia. 19:159–61. DOI: 10.1038/sj.leu.2403534. PMID: 15457187.
Article11. Drexler HG, Minowada J. 1998; History and classification of human leukemia-lymphoma cell lines. Leuk Lymphoma. 31:305–16. DOI: 10.3109/10428199809059223. PMID: 9869194.
Article12. Spira G, Aman P, Koide N, Lundin G, Klein G, Hall K. 1981; Cell-surface immunoglobulin and insulin receptor expression in an EBV-negative lymphoma cell line and its EBV-converted sublines. J Immunol. 126:122–6. PMID: 6256436.13. Tao Q, Young LS, Woodman CB, Murray PG. 2006; Epstein-Barr virus (EBV) and its associated human cancers--genetics, epigenetics, pathobiology and novel therapeutics. Front Biosci. 11:2672–713. DOI: 10.2741/2000. PMID: 16720343.14. Chen X, Kamranvar SA, Masucci MG. 2016; Oxidative stress enables Epstein-Barr virus-induced B-cell transformation by posttranscriptional regulation of viral and cellular growth-promoting factors. Oncogene. 35:3807–16. DOI: 10.1038/onc.2015.450. PMID: 26592445.
Article15. Kgatle MM, Spearman CW, Kalla AA, Hairwadzi HN. 2017; DNA oncogenic virus-induced oxidative stress, genomic damage, and aberrant epigenetic alterations. Oxid Med Cell Longev. 2017:3179421. DOI: 10.1155/2017/3179421. PMID: 28740569. PMCID: PMC5504953.
Article16. Brandes RP, Weissmann N, Schröder K. 2014; Nox family NADPH oxidases: molecular mechanisms of activation. Free Radic Biol Med. 76:208–26. DOI: 10.1016/j.freeradbiomed.2014.07.046. PMID: 25157786.
Article17. Bonner MY, Arbiser JL. 2012; Targeting NADPH oxidases for the treatment of cancer and inflammation. Cell Mol Life Sci. 69:2435–42. DOI: 10.1007/s00018-012-1017-2. PMID: 22581366. PMCID: PMC4405775.
Article18. Cho SY, Kim JS, Eun HS, Kang SH, Lee ES, Kim SH, Sung JK, Lee BS, Jeong HY, Moon HS. 2018; Expression of Nox family genes and their clinical significance in colorectal cancer. Dig Dis Sci. 63:2332–40. DOI: 10.1007/s10620-018-5121-5. PMID: 29781053.
Article19. Leung EL, Fan XX, Wong MP, Jiang ZH, Liu ZQ, Yao XJ, Lu LL, Zhou YL, Yau LF, Tin VP, Liu L. 2016; Targeting tyrosine kinase inhibitor-resistant non-small cell lung cancer by inducing epidermal growth factor receptor degradation via methionine 790 oxidation. Antioxid Redox Signal. 24:263–79. DOI: 10.1089/ars.2015.6420. PMID: 26528827. PMCID: PMC4753639.20. Ding Y, Zhu W, Sun R, Yuan G, Zhang D, Fan Y, Sun J. 2015; Diphenylene iodonium interferes with cell cycle progression and induces apoptosis by modulating NAD(P)H oxidase/ROS/cell cycle regulatory pathways in Burkitt's lymphoma cells. Oncol Rep. 33:1434–42. DOI: 10.3892/or.2015.3726. PMID: 25591797.
Article21. Klingenberg M, Becker J, Eberth S, Kube D, Wilting J. 2014; The NADPH oxidase inhibitor imipramine-blue in the treatment of Burkitt lymphoma. Mol Cancer Ther. 13:833–41. DOI: 10.1158/1535-7163.MCT-13-0688. PMID: 24482381.
Article22. Suvarna V, Singh V, Murahari M. 2019; Current overview on the clinical update of Bcl-2 anti-apoptotic inhibitors for cancer therapy. Eur J Pharmacol. 862:172655. DOI: 10.1016/j.ejphar.2019.172655. PMID: 31494078.
Article23. Knight T, Luedtke D, Edwards H, Taub JW, Ge Y. 2019; A delicate balance- the BCL-2 family and its role in apoptosis, oncogenesis, and cancer therapeutics. Biochem Pharmacol. 162:250–61. DOI: 10.1016/j.bcp.2019.01.015. PMID: 30668936.24. Singh R, Letai A, Sarosiek K. 2019; Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins. Nat Rev Mol Cell Biol. 20:175–93. DOI: 10.1038/s41580-018-0089-8. PMID: 30655609. PMCID: PMC7325303.
Article25. Huska JD, Lamb HM, Hardwick JM. 2019; Overview of BCL-2 family proteins and therapeutic potentials. Methods Mol Biol. 1877:1–21. DOI: 10.1007/978-1-4939-8861-7_1. PMID: 30535995.
Article26. Peña-Blanco A, García-Sáez AJ. 2018; Bax, Bak and beyond- mitochondrial performance in apoptosis. FEBS J. 285:416–31. DOI: 10.1111/febs.14186. PMID: 28755482.27. Ugarte-Uribe B, García-Sáez AJ. 2017; Apoptotic foci at mitochondria: in and around Bax pores. Philos Trans R Soc Lond B Biol Sci. 372:20160217. DOI: 10.1098/rstb.2016.0217. PMID: 28630156. PMCID: PMC5483519.
Article28. Luna-Vargas MPA, Chipuk JE. 2016; Physiological and pharmacological control of BAK, BAX, and beyond. Trends Cell Biol. 26:906–17. DOI: 10.1016/j.tcb.2016.07.002. PMID: 27498846. PMCID: PMC5118054.
Article29. Cerimele F, Battle T, Lynch R, Frank DA, Murad E, Cohen C, Macaron N, Sixbey J, Smith K, Watnick RS, Eliopoulos A, Shehata B, Arbiser JL. 2005; Reactive oxygen signaling and MAPK activation distinguish Epstein-Barr virus (EBV)-positive versus EBV-negative Burkitt's lymphoma. Proc Natl Acad Sci U S A. 102:175–9. DOI: 10.1073/pnas.0408381102. PMID: 15611471. PMCID: PMC544042.
Article30. Williams VM, Filippova M, Filippov V, Payne KJ, Duerksen-Hughes P. 2014; Human papillomavirus type 16 E6* induces oxidative stress and DNA damage. J Virol. 88:6751–61. DOI: 10.1128/JVI.03355-13. PMID: 24696478. PMCID: PMC4054338.
Article31. Fu N, Yao H, Nan Y, Qiao L. 2017; Role of oxidative stress in Hepatitis C virus induced hepatocellular carcinoma. Curr Cancer Drug Targets. 17:498–504. DOI: 10.2174/1568009616666160926124043. PMID: 27677955.
Article32. Sun J, Hu C, Zhu Y, Sun R, Fang Y, Fan Y, Xu F. 2015; LMP1 increases expression of NADPH oxidase (Nox) and its regulatory subunit p22 in NP69 nasopharyngeal cells and makes them sensitive to a treatment by a Nox inhibitor. PLoS One. 10:e0134896. DOI: 10.1371/journal.pone.0134896. PMID: 26244812. PMCID: PMC4526464.
Article33. Herr I, Debatin KM. 2001; Cellular stress response and apoptosis in cancer therapy. Blood. 98:2603–14. DOI: 10.1182/blood.V98.9.2603. PMID: 11675328.
Article34. Wang C, Youle RJ. 2009; The role of mitochondria in apoptosis*. Annu Rev Genet. 43:95–118. DOI: 10.1146/annurev-genet-102108-134850. PMID: 19659442. PMCID: PMC4762029.
Article35. Brenner D, Mak TW. 2009; Mitochondrial cell death effectors. Curr Opin Cell Biol. 21:871–7. DOI: 10.1016/j.ceb.2009.09.004. PMID: 19822411.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recent trends in two-photon auto-fluorescence lifetime imaging (2P-FLIM) and its biomedical applications
- Novel Therapeutic Target of Non-alcoholic Fatty Liver Disease: Nicotinamide Adenine Dinucleotide Phosphate Oxidase 2 and Reactive Oxygen Species in Pro-inflammatory Macrophages
- Inhibitory Effect of Apocynin on Proteasome Inhibition-Induced Apoptosis in Differentiated PC12 Cells
- Effects of oxidative stress on endothelial modulation of contractions in aorta from renal hypertensive rats
- Inhibition of Nicotinamide Phosphoribosyltransferase Induces Apoptosis in Estrogen Receptor-Positive MCF-7 Breast Cancer Cells