J Korean Med Sci.
2020 Dec;35(48):e371. 10.3346/jkms.2020.35.e371.
Cell-type-specific Modulation of Nonhomologous End Joining of Gamma Ray-induced DNA Double-strand Breaks by cAMP Signaling in Human Cancer Cells
- Affiliations
-
- 1Department of Biochemistry and Molecular Biology, Department of Biomedical Sciences, and Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea
- KMID: 2509524
- DOI: http://doi.org/10.3346/jkms.2020.35.e371
Abstract
- Background
Cyclic AMP (cAMP) signaling is activated by various hormones and neurotransmitters and regulates numerous physiological phenomena, including energy metabolism, gene expression, and proliferation. cAMP signaling plays a role in the repair of DNA damage, but its specific function is inconsistent in the literature. The present study aimed to investigate the mechanism of the different roles of cAMP signaling in DNA repair by analyzing the cell-type differences in the modulation of DNA repair by cAMP signaling following γ-ray irradiation.
Methods
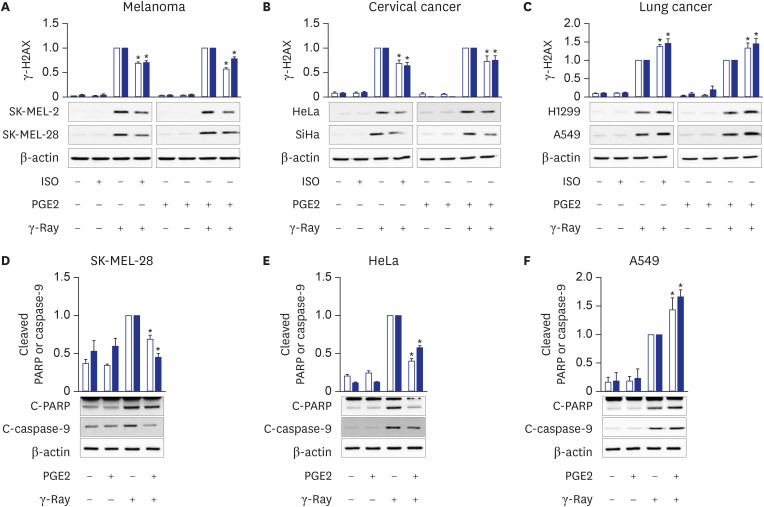
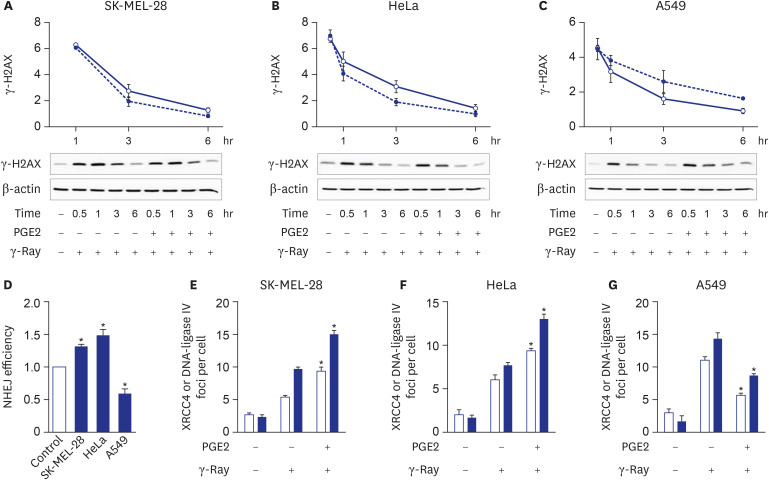
cAMP signaling was activated in human malignant melanoma cells (SK-MEL-2 and SK-MEL-28), human uterine cervical cancer cells (HeLa and SiHa) and human non-small cell lung cancer cells (H1299 and A549) by expressing a constitutively active mutant of the long-form stimulatory α subunit of GTP-binding protein or by treating with isoproterenol and prostaglandin E2 before γ-ray irradiation. DNA damage was quantitated by western blot analysis of γ-H2AX, and non-homologous end joining (NHEJ) was assessed by fluorescent reporter plasmid repair assay and immunofluorescence of microscopic foci of XRCC4 and DNA-ligase IV.
Results
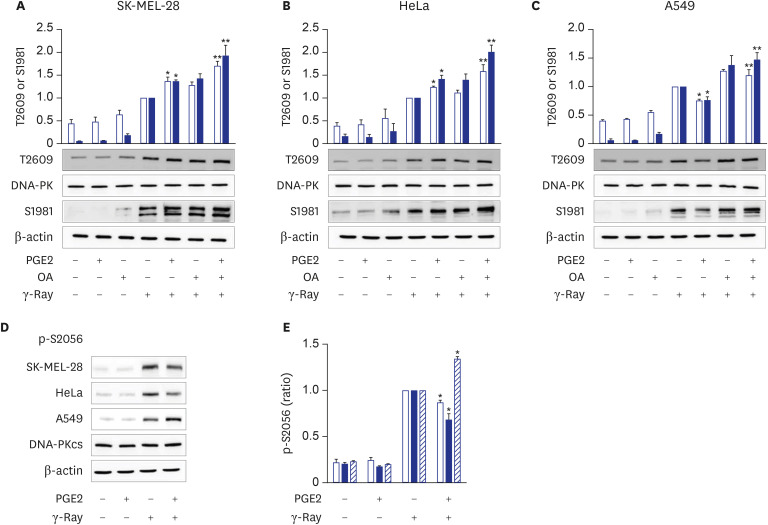
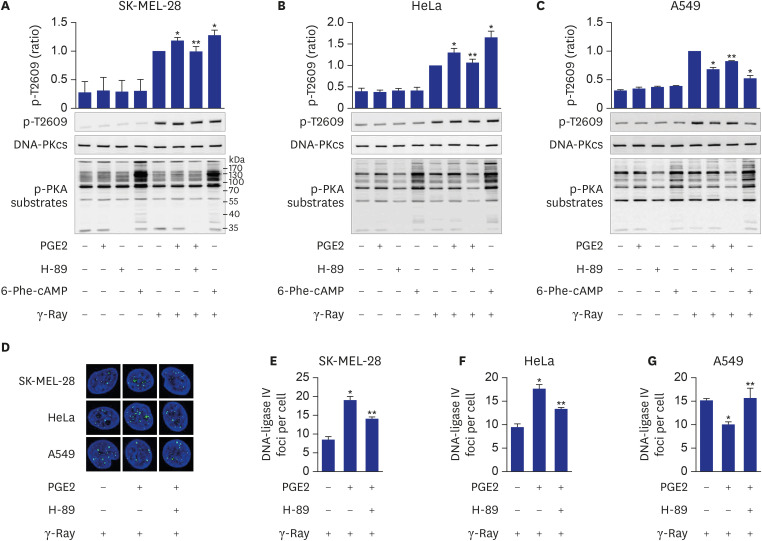
cAMP signaling modulated DNA damage, apoptosis and the NHEJ repair following γ-ray irradiation differently depending upon the cell type. cAMP signaling regulated the phosphorylation of DNA-dependent protein kinase catalytic subunit (DNA-PKcs) at Ser2056 and Thr2609 in cell-type-specific manners following γ-ray irradiation, an activity that was mediated by protein kinase A.
Conclusion
cAMP signaling modulates the NHEJ repair of γ-ray-induced DNA damage in melanoma cells, uterine cervical cancer cells and lung cancer cells in a cell-typespecific manner, and the modulation is likely mediated by protein kinase A-dependent phosphorylation of DNA-PKcs. This study suggests that cell- and tissue-specific modulation of DNA damage repair by cAMP signaling may contribute to improve the therapeutic efficiency of radiation therapy.
Keyword
Figure
Reference
-
1. Wahlang B, McClain C, Barve S, Gobejishvili L. Role of cAMP and phosphodiesterase signaling in liver health and disease. Cell Signal. 2018; 49:105–115. PMID: 29902522.
Article2. Gold MG, Gonen T, Scott JD. Local cAMP signaling in disease at a glance. J Cell Sci. 2013; 126(Pt 20):4537–4543. PMID: 24124191.
Article3. Hussain M, Tang F, Liu J, Zhang J, Javeed A. Dichotomous role of protein kinase A type I (PKAI) in the tumor microenvironment: a potential target for ‘two-in-one’ cancer chemoimmunotherapeutics. Cancer Lett. 2015; 369(1):9–19. PMID: 26276720.
Article4. Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009; 461(7267):1071–1078. PMID: 19847258.
Article5. Mladenov E, Magin S, Soni A, Iliakis G. DNA double-strand-break repair in higher eukaryotes and its role in genomic instability and cancer: Cell cycle and proliferation-dependent regulation. Semin Cancer Biol. 2016; 37-38:51–64. PMID: 27016036.
Article6. Davis AJ, Chen DJ. DNA double strand break repair via non-homologous end-joining. Transl Cancer Res. 2013; 2(3):130–143. PMID: 24000320.7. Liu B, Cvijic ME, Jetzt A, Chin KV. Cisplatin resistance and regulation of DNA repair in cAMP-dependent protein kinase mutants. Cell Growth Differ. 1996; 7(8):1105–1112. PMID: 8853907.8. Kadekaro AL, Kavanagh R, Kanto H, Terzieva S, Hauser J, Kobayashi N, et al. alpha-Melanocortin and endothelin-1 activate antiapoptotic pathways and reduce DNA damage in human melanocytes. Cancer Res. 2005; 65(10):4292–4299. PMID: 15899821.9. Kadekaro AL, Chen J, Yang J, Chen S, Jameson J, Swope VB, et al. Alpha-melanocyte-stimulating hormone suppresses oxidative stress through a p53-mediated signaling pathway in human melanocytes. Mol Cancer Res. 2012; 10(6):778–786. PMID: 22622028.
Article10. Cho EA, Juhnn YS. The cAMP signaling system inhibits the repair of γ-ray-induced DNA damage by promoting Epac1-mediated proteasomal degradation of XRCC1 protein in human lung cancer cells. Biochem Biophys Res Commun. 2012; 422(2):256–262. PMID: 22575451.
Article11. Noh SE, Juhnn YS. Inhibition of non-homologous end joining of gamma ray-induced DNA double-strand breaks by cAMP signaling in lung cancer cells. Sci Rep. 2020; 10(1):14455. PMID: 32879366.
Article12. Seluanov A, Mittelman D, Pereira-Smith OM, Wilson JH, Gorbunova V. DNA end joining becomes less efficient and more error-prone during cellular senescence. Proc Natl Acad Sci U S A. 2004; 101(20):7624–7629. PMID: 15123826.
Article13. Dumont JE, Jauniaux JC, Roger PP. The cyclic AMP-mediated stimulation of cell proliferation. Trends Biochem Sci. 1989; 14(2):67–71. PMID: 2539663.
Article14. Insel PA, Zhang L, Murray F, Yokouchi H, Zambon AC. Cyclic AMP is both a pro-apoptotic and anti-apoptotic second messenger. Acta Physiol (Oxf). 2012; 204(2):277–287. PMID: 21385327.
Article15. Ladilov Y, Appukuttan A. Role of soluble adenylyl cyclase in cell death and growth. Biochim Biophys Acta. 2014; 1842(12 Pt B):2646–2655. PMID: 25010002.
Article16. Jarrett SG, Wolf Horrell EM, Christian PA, Vanover JC, Boulanger MC, Zou Y, et al. PKA-mediated phosphorylation of ATR promotes recruitment of XPA to UV-induced DNA damage. Mol Cell. 2014; 54(6):999–1011. PMID: 24950377.
Article17. Wolf Horrell EM, Boulanger MC, D'Orazio JA. Melanocortin 1 Receptor: Structure, Function, and Regulation. Front Genet. 2016; 7:95. PMID: 27303435.
Article18. Jarrett SG, Carter KM, Shelton BJ, D'Orazio JA. The melanocortin signaling cAMP axis accelerates repair and reduces mutagenesis of platinum-induced DNA damage. Sci Rep. 2017; 7(1):11708. PMID: 28916831.
Article19. Castejón-Griñán M, Herraiz C, Olivares C, Jiménez-Cervantes C, García-Borrón JC. cAMP-independent non-pigmentary actions of variant melanocortin 1 receptor: AKT-mediated activation of protective responses to oxidative DNA damage. Oncogene. 2018; 37(27):3631–3646. PMID: 29622793.
Article20. Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007; 370(9590):890–907. PMID: 17826171.
Article21. Sales KJ, Katz AA, Howard B, Soeters RP, Millar RP, Jabbour HN. Cyclooxygenase-1 is up-regulated in cervical carcinomas: autocrine/paracrine regulation of cyclooxygenase-2, prostaglandin e receptors, and angiogenic factors by cyclooxygenase-1. Cancer Res. 2002; 62(2):424–432. PMID: 11809691.22. Oh JM, Kim SH, Lee YI, Seo M, Kim SY, Song YS, et al. Human papillomavirus E5 protein induces expression of the EP4 subtype of prostaglandin E2 receptor in cyclic AMP response element-dependent pathways in cervical cancer cells. Carcinogenesis. 2009; 30(1):141–149. PMID: 18849297.
Article23. Adefuye A, Sales K. Regulation of inflammatory pathways in cancer and infectious disease of the cervix. Scientifica (Cairo). 2012; 2012:548150. PMID: 24278714.
Article24. Wagner W, Ciszewski WM, Kania KD. L- and D-lactate enhance DNA repair and modulate the resistance of cervical carcinoma cells to anticancer drugs via histone deacetylase inhibition and hydroxycarboxylic acid receptor 1 activation. Cell Commun Signal. 2015; 13(1):36. PMID: 26208712.
Article25. Morland C, Lauritzen KH, Puchades M, Holm-Hansen S, Andersson K, Gjedde A, et al. The lactate receptor, G-protein-coupled receptor 81/hydroxycarboxylic acid receptor 1: Expression and action in brain. J Neurosci Res. 2015; 93(7):1045–1055. PMID: 25881750.
Article26. Cho EA, Oh JM, Kim SY, Kim Y, Juhnn YS. Heterotrimeric stimulatory GTP-binding proteins inhibit cisplatin-induced apoptosis by increasing X-linked inhibitor of apoptosis protein expression in cervical cancer cells. Cancer Sci. 2011; 102(4):837–844. PMID: 21255191.
Article27. Cho EA, Kim EJ, Kwak SJ, Juhnn YS. cAMP signaling inhibits radiation-induced ATM phosphorylation leading to the augmentation of apoptosis in human lung cancer cells. Mol Cancer. 2014; 13(1):36. PMID: 24568192.
Article28. Jette N, Lees-Miller SP. The DNA-dependent protein kinase: A multifunctional protein kinase with roles in DNA double strand break repair and mitosis. Prog Biophys Mol Biol. 2015; 117(2-3):194–205. PMID: 25550082.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pathways and genes of DNA double-strand break repair associated with head and neck cancer
- Aberrant DNA Double-strand Break Repair Threads in Breast Carcinoma: Orchestrating Genomic Insult Survival
- Evaluation of DNA Double Strand Breaks in Human and Mouse Lymphocyte Following gamma-Irradiation
- Silibinin Radiosensitizes EGF Receptor-knockdown Prostate Cancer Cells by Attenuating DNA Repair Pathways
- The Expressions of p53, gamma-H(2)AX and Ku70/Ku80 That was Caused by Hydronephrosis in the Kidney of Rats