Int J Stem Cells.
2020 Nov;13(3):335-341. 10.15283/ijsc20091.
Stem Cells for the Regeneration of Tendon and Ligament: A Perspective
- Affiliations
-
- 1Research Institute for Integrative Regenerative Biomedical Engineering, Dongguk University, Goyang, Korea
- KMID: 2508907
- DOI: http://doi.org/10.15283/ijsc20091
Abstract
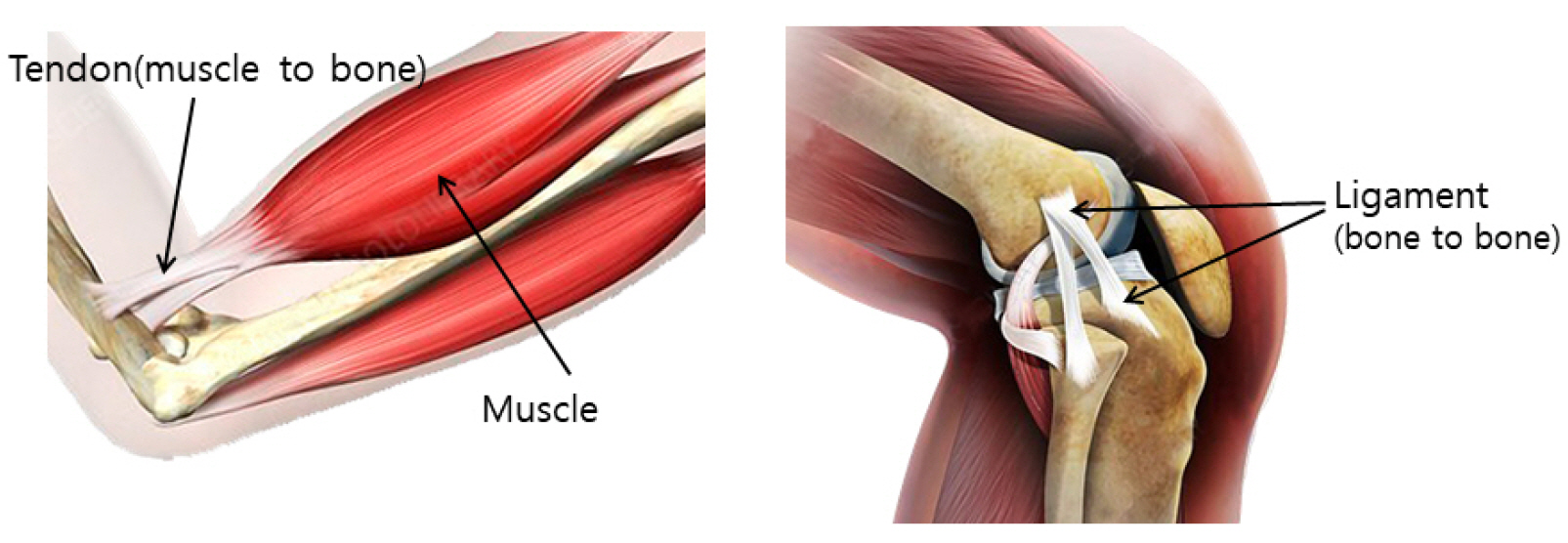
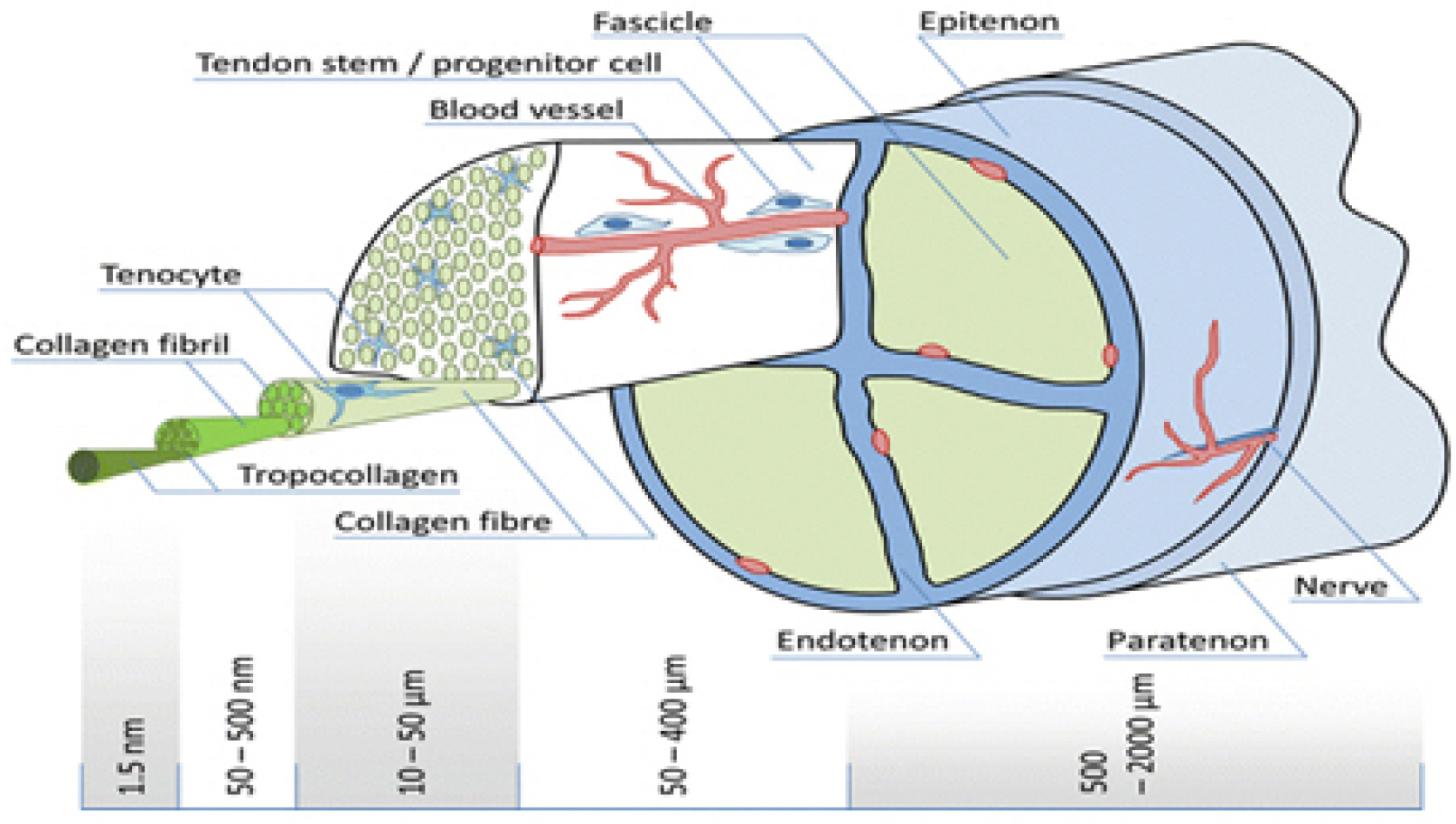
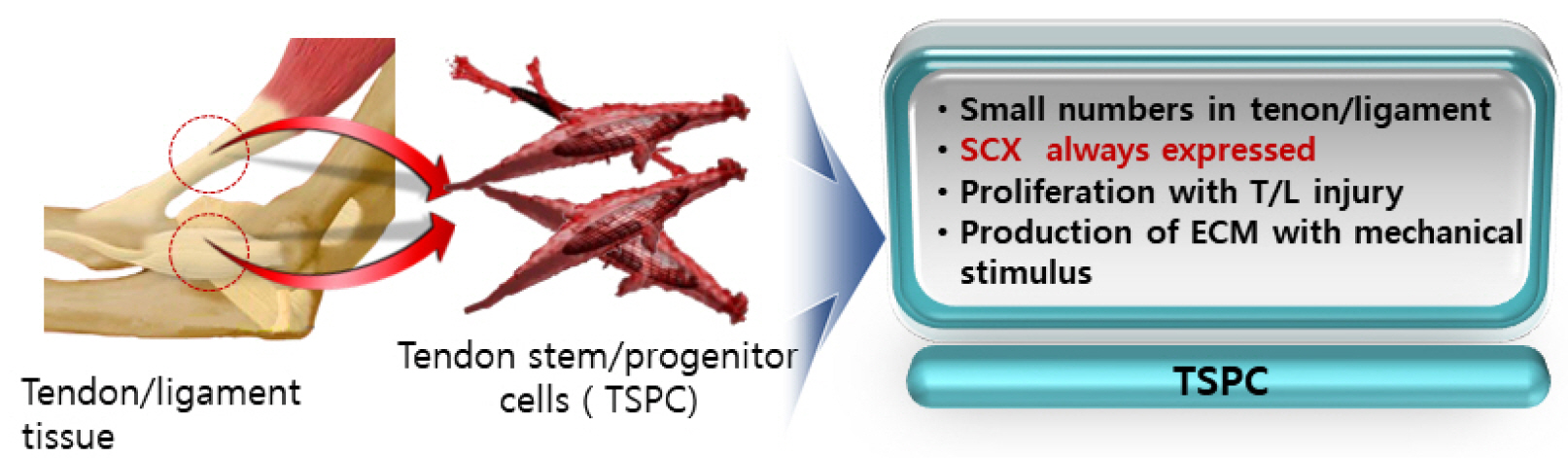
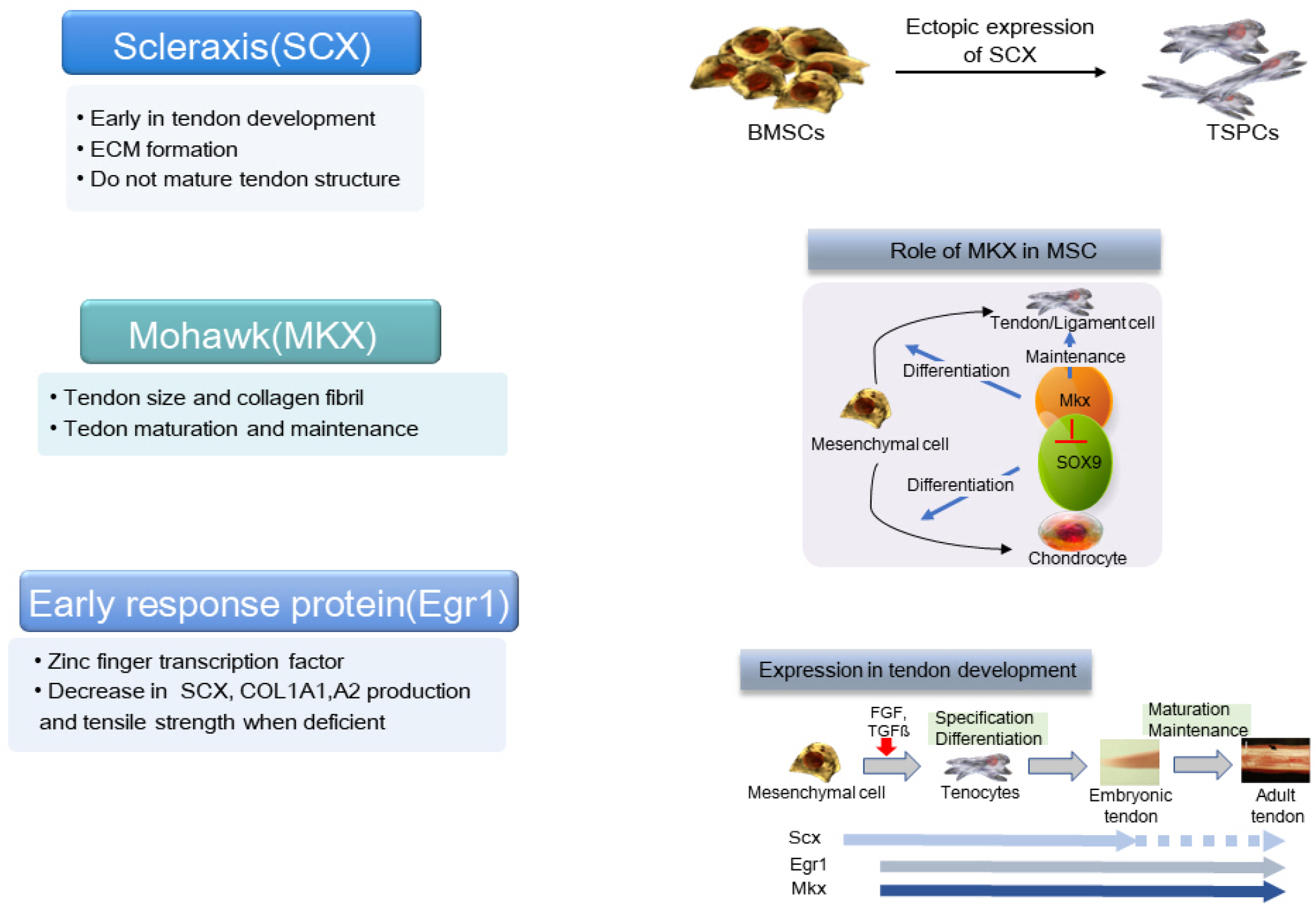
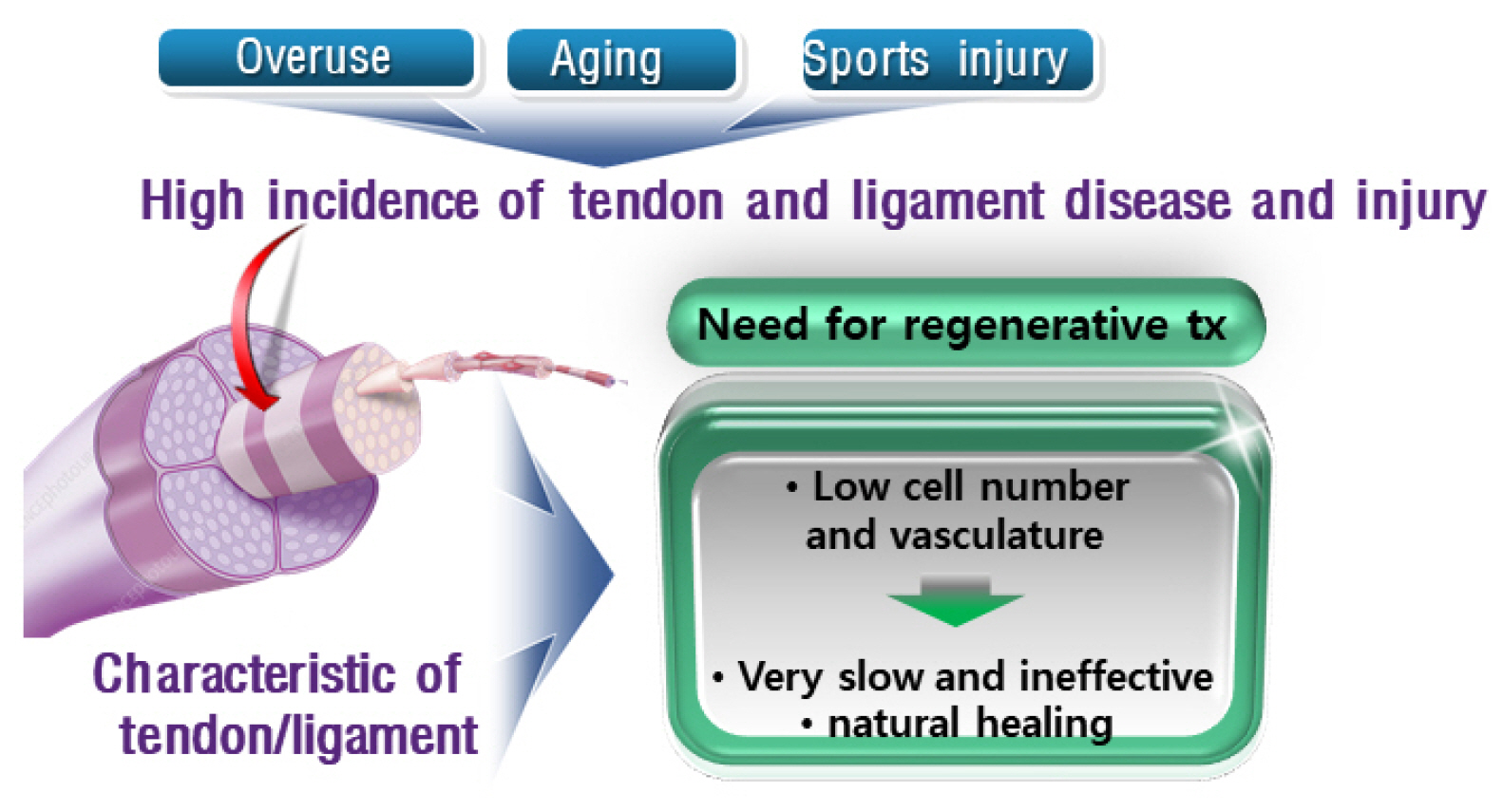
- Tendons are structures that connect muscles to the bones in our body and transmit the force generated by contraction of the muscles to the bones. Ligaments are structures that connect bones to bones, with histological properties similar to tendons. In tendon and ligament tissue, there are very small amounts of cells similar to mesenchymal stem cells (MSCs) called tendon stem/progenitor cells (TSPCs), or tenogenic stem cells. While the role of specific growth factors and transcription factors is well established in the osteogenic and chondrogenic differentiation of stem cells, a consensus has not been established for tenogenic differentiation. Injuries to tendons and ligaments are very common, but natural healing is very slow and inefficient due to limited vascularization. Currently, there is no adequate method for restoring extensive tendon or ligament defects. Procedures addressing the unmet need for regeneration of these tissues are needed. In this review, the current knowledge, as well as the authors’ ideas and perspective on stem cell and regenerative medicine for tendon and ligament defects are presented.
Figure
Reference
-
References
1. Docheva D, Müller SA, Majewski M, Evans CH. 2015; Biologics for tendon repair. Adv Drug Deliv Rev. 84:222–239. DOI: 10.1016/j.addr.2014.11.015. PMID: 25446135. PMCID: PMC4519231.
Article2. Alberton P, Dex S, Popov C, Shukunami C, Schieker M, Docheva D. 2015; Loss of tenomodulin results in reduced self-renewal and augmented senescence of tendon stem/progenitor cells. Stem Cells Dev. 24:597–609. DOI: 10.1089/scd.2014.0314. PMID: 25351164. PMCID: PMC4333258.
Article3. Figueroa D, Figueroa F, Calvo R. 2016; Patellar tendinopathy: diagnosis and treatment. J Am Acad Orthop Surg. 24:e184–e192. DOI: 10.5435/JAAOS-D-15-00703. PMID: 27855131.4. Huang AH, Lu HH, Schweitzer R. 2015; Molecular regulation of tendon cell fate during development. J Orthop Res. 33:800–812. DOI: 10.1002/jor.22834. PMID: 25664867.
Article5. Walia B, Huang AH. 2019; Tendon stem progenitor cells: understanding the biology to inform therapeutic strategies for tendon repair. J Orthop Res. 37:1270–1280. DOI: 10.1002/jor.24156. PMID: 30270569. PMCID: PMC6823601.
Article6. Alberton P, Popov C, Prägert M, Kohler J, Shukunami C, Schieker M, Docheva D. 2012; Conversion of human bone marrow-derived mesenchymal stem cells into tendon progenitor cells by ectopic expression of scleraxis. Stem Cells Dev. 21:846–858. DOI: 10.1089/scd.2011.0150. PMID: 21988170. PMCID: PMC3315756.
Article7. Asahara H, Inui M, Lotz MK. 2017; Tendons and ligaments: connecting developmental biology to musculoskeletal disease pathogenesis. J Bone Miner Res. 32:1773–1782. DOI: 10.1002/jbmr.3199. PMID: 28621492. PMCID: PMC5585011.
Article8. Otabe K, Nakahara H, Hasegawa A, Matsukawa T, Ayabe F, Onizuka N, Inui M, Takada S, Ito Y, Sekiya I, Muneta T, Lotz M, Asahara H. 2015; Transcription factor Mohawk controls tenogenic differentiation of bone marrow mesenchy-mal stem cells in vitro and in vivo. J Orthop Res. 33:1–8. DOI: 10.1002/jor.22750. PMID: 25312837. PMCID: PMC4294629.
Article9. Liu H, Zhang C, Zhu S, Lu P, Zhu T, Gong X, Zhang Z, Hu J, Yin Z, Heng BC, Chen X, Ouyang HW. 2015; Mohawk promotes the tenogenesis of mesenchymal stem cells through activation of the TGFβ signaling pathway. Stem Cells. 33:443–455. DOI: 10.1002/stem.1866. PMID: 25332192.
Article10. Li X, Pongkitwitoon S, Lu H, Lee C, Gelberman R, Thomopoulos S. 2019; CTGF induces tenogenic differentiation and proliferation of adipose-derived stromal cells. J Orthop Res. 37:574–582. DOI: 10.1002/jor.24248. PMID: 30756417. PMCID: PMC6467286.
Article11. Lee CH, Lee FY, Tarafder S, Kao K, Jun Y, Yang G, Mao JJ. 2015; Harnessing endogenous stem/progenitor cells for tendon regeneration. J Clin Invest. 125:2690–2701. DOI: 10.1172/JCI81589. PMID: 26053662. PMCID: PMC4563693.
Article12. Yoshikawa Y, Abrahamsson SO. 2001; Dose-related cellular effects of platelet-derived growth factor-BB differ in various types of rabbit tendons in vitro. Acta Orthop Scand. 72:287–292. DOI: 10.1080/00016470152846646. PMID: 11480607.
Article13. Thomopoulos S, Harwood FL, Silva MJ, Amiel D, Gelberman RH. 2005; Effect of several growth factors on canine flexor tendon fibroblast proliferation and collagen synthesis in vitro. J Hand Surg Am. 30:441–447. DOI: 10.1016/j.jhsa.2004.12.006. PMID: 15925149.
Article14. Pryce BA, Watson SS, Murchison ND, Staverosky JA, Dünker N, Schweitzer R. 2009; Recruitment and maintenance of tendon progenitors by TGFbeta signaling are essential for tendon formation. Development. 136:1351–1361. DOI: 10.1242/dev.027342. PMID: 19304887. PMCID: PMC2687466.
Article15. Havis E, Bonnin MA, Olivera-Martinez I, Nazaret N, Ruggiu M, Weibel J, Durand C, Guerquin MJ, Bonod-Bidaud C, Ruggiero F, Schweitzer R, Duprez D. 2014; Transcrip-tomic analysis of mouse limb tendon cells during develop-ment. Development. 141:3683–3696. DOI: 10.1242/dev.108654. PMID: 25249460.
Article16. Mendias CL, Bakhurin KI, Faulkner JA. 2008; Tendons of myostatin-deficient mice are small, brittle, and hypocellular. Proc Natl Acad Sci U S A. 105:388–393. DOI: 10.1073/pnas.0707069105. PMID: 18162552. PMCID: PMC2224222.
Article17. Schweitzer R, Chyung JH, Murtaugh LC, Brent AE, Rosen V, Olson EN, Lassar A, Tabin CJ. 2001; Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development. 128:3855–3866. PMID: 11585810.
Article18. Bénazet JD, Pignatti E, Nugent A, Unal E, Laurent F, Zeller R. 2012; Smad4 is required to induce digit ray primordia and to initiate the aggregation and differentiation of chondrogenic progenitors in mouse limb buds. Development. 139:4250–4260. DOI: 10.1242/dev.084822. PMID: 23034633.
Article19. Rui YF, Lui PP, Wong YM, Tan Q, Chan KM. 2013; BMP-2 stimulated non-tenogenic differentiation and promoted proteoglycan deposition of tendon-derived stem cells (TDSCs) in vitro. J Orthop Res. 31:746–753. DOI: 10.1002/jor.22290. PMID: 23238867.
Article20. Prabhath A, Vernekar VN, Sanchez E, Laurencin CT. 2018; Growth factor delivery strategies for rotator cuff repair and regeneration. Int J Pharm. 544:358–371. DOI: 10.1016/j.ijpharm.2018.01.006. PMID: 29317260.
Article21. Haddad-Weber M, Prager P, Kunz M, Seefried L, Jakob F, Murray MM, Evans CH, Nöth U, Steinert AF. 2010; BMP12 and BMP13 gene transfer induce ligamentogenic differentiation in mesenchymal progenitor and anterior cruciate ligament cells. Cytotherapy. 12:505–513. DOI: 10.3109/14653241003709652. PMID: 20334610. PMCID: PMC3580941.
Article22. Wang D, Jiang X, Lu A, Tu M, Huang W, Huang P. 2018; BMP14 induces tenogenic differentiation of bone marrow mesenchymal stem cells in vitro. Exp Ther Med. 16:1165–1174. DOI: 10.3892/etm.2018.6293. PMID: 30116367. PMCID: PMC6090266.23. Zhang YJ, Chen X, Li G, Chan KM, Heng BC, Yin Z, Ouyang HW. 2018; Concise review: stem cell fate guided by bioactive molecules for tendon regeneration. Stem Cells Transl Med. 7:404–414. DOI: 10.1002/sctm.17-0206. PMID: 29573225. PMCID: PMC5905226.
Article24. Holladay C, Abbah SA, O'Dowd C, Pandit A, Zeugolis DI. 2016; Preferential tendon stem cell response to growth factor supplementation. J Tissue Eng Regen Med. 10:783–798. DOI: 10.1002/term.1852. PMID: 24474722.
Article25. Keller TC, Hogan MV, Kesturu G, James R, Balian G, Chhabra AB. 2011; Growth/differentiation factor-5 modulates the synthesis and expression of extracellular matrix and cell-adhesion-related molecules of rat Achilles tendon fibro-blasts. Connect Tissue Res. 52:353–364. DOI: 10.3109/03008207.2010.534208. PMID: 21250863.
Article26. Hogan M, Girish K, James R, Balian G, Hurwitz S, Chhabra AB. 2011; Growth differentiation factor-5 regulation of extracellular matrix gene expression in murine tendon fibroblasts. J Tissue Eng Regen Med. 5:191–200. DOI: 10.1002/term.304. PMID: 20653042.
Article27. Liu J, Tao X, Chen L, Han W, Zhou Y, Tang K. 2015; CTGF positively regulates BMP12 induced tenogenic differentia-tion of tendon stem cells and signaling. Cell Physiol Biochem. 35:1831–1845. DOI: 10.1159/000373994. PMID: 25833297.
Article28. Xu K, Sun Y, Kh Al-Ani M, Wang C, Sha Y, Sung KP, Dong N, Qiu X, Yang L. 2018; Synergistic promoting effects of bone morphogenetic protein 12/connective tissue growth factor on functional differentiation of tendon derived stem cells and patellar tendon window defect regeneration. J Biomech. 66:95–102. DOI: 10.1016/j.jbiomech.2017.11.004. PMID: 29174694.
Article29. Popov C, Burggraf M, Kreja L, Ignatius A, Schieker M, Docheva D. 2015; Mechanical stimulation of human tendon stem/progenitor cells results in upregulation of matrix proteins, integrins and MMPs, and activation of p38 and ERK1/2 kinases. BMC Mol Biol. 16:6. DOI: 10.1186/s12867-015-0036-6. PMID: 25880261. PMCID: PMC4373449.
Article30. Wang T, Thien C, Wang C, Ni M, Gao J, Wang A, Jiang Q, Tuan RS, Zheng Q, Zheng MH. 2018; 3D uniaxial mechanical stimulation induces tenogenic differentiation of tendon-derived stem cells through a PI3K/AKT signaling pathway. FASEB J. 32:4804–4814. DOI: 10.1096/fj.201701384R. PMID: 29596022.
Article31. Yao L, Bestwick CS, Bestwick LA, Maffulli N, Aspden RM. 2006; Phenotypic drift in human tenocyte culture. Tissue Eng. 12:1843–1849. DOI: 10.1089/ten.2006.12.1843. PMID: 16889514.
Article32. Tan Q, Lui PP, Rui YF. 2012; Effect of in vitro passaging on the stem cell-related properties of tendon-derived stem cells-implications in tissue engineering. Stem Cells Dev. 21:790–800. DOI: 10.1089/scd.2011.0160. PMID: 21627568. PMCID: PMC3295857.
Article33. Lee WY, Lui PP, Rui YF. 2012; Hypoxia-mediated efficient expansion of human tendon-derived stem cells in vitro. Tissue Eng Part A. 18:484–498. DOI: 10.1089/ten.tea.2011.0130. PMID: 21943340. PMCID: PMC3286812.
Article34. Zhang J, Wang JH. 2013; Human tendon stem cells better maintain their stemness in hypoxic culture conditions. PLoS One. 8:e61424. DOI: 10.1371/journal.pone.0061424. PMID: 23613849. PMCID: PMC3629026.
Article35. Yu Y, Lin L, Zhou Y, Lu X, Shao X, Lin C, Yu K, Zhang X, Hong J, Chen Y. 2017; Effect of hypoxia on self-renewal capacity and differentiation in human tendon-derived stem cells. Med Sci Monit. 23:1334–1339. DOI: 10.12659/MSM.903892. PMID: 28302994. PMCID: PMC5367841.
Article36. Komura S, Satake T, Goto A, Aoki H, Shibata H, Ito K, Hirakawa A, Yamada Y, Akiyama H. 2020; Induced pluripotent stem cell-derived tenocyte-like cells promote the regene-ration of injured tendons in mice. Sci Rep. 10:3992. DOI: 10.1038/s41598-020-61063-6. PMID: 32132649. PMCID: PMC7055210.
Article37. McClellan A, Paterson YZ, Paillot R, Guest DJ. 2019; Equine fetal, adult, and embryonic stem cell-derived tenocytes are all immune privileged but exhibit different immune suppressive properties in vitro. Stem Cells Dev. 28:1413–1423. DOI: 10.1089/scd.2019.0120. PMID: 31507234.
Article38. Dale TP, Mazher S, Webb WR, Zhou J, Maffulli N, Chen GQ, El Haj AJ, Forsyth NR. 2018; Tenogenic differentiation of human embryonic stem cells. Tissue Eng Part A. 24:361–368. DOI: 10.1089/ten.tea.2017.0017. PMID: 28548630.
Article39. Ljungqvist A, Schwellnus MP, Bachl N, Collins M, Cook J, Khan KM, Maffulli N, Pitsiladis Y, Riley G, Golspink G, Venter D, Derman EW, Engebretsen L, Volpi P. 2008; International Olympic Committee consensus statement: molecular basis of connective tissue and muscle injuries in sport. Clin Sports Med. 27:231–239. DOI: 10.1016/j.csm.2007.10.007. PMID: 18206577.
Article40. Singh N. 2017; International epidemiology of anterior cruciate ligament injuries. Orthop Res Online J. 1:94–96. DOI: 10.31031/OPROJ.2018.01.000525.
Article41. Khatri C, Ahmed I, Parsons H, Smith NA, Lawrence TM, Modi CS, Drew SJ, Bhabra G, Parsons NR, Underwood M, Metcalfe AJ. 2019; The natural history of full-thickness rotator cuff tears in randomized controlled trials: a systematic review and meta-analysis. Am J Sports Med. 47:1734–1743. DOI: 10.1177/0363546518780694. PMID: 29963905.
Article42. Reilly P, Macleod I, Macfarlane R, Windley J, Emery RJ. 2006; Dead men and radiologists don't lie: a review of cadaveric and radiological studies of rotator cuff tear prevalence. Ann R Coll Surg Engl. 88:116–121. DOI: 10.1308/003588406X94968. PMID: 16551396. PMCID: PMC1964063.
Article43. Chalmers PN, Ross H, Granger E, Presson AP, Zhang C, Tashjian RZ. 2018; The effect of rotator cuff repair on natural history: a systematic review of intermediate to long-term outcomes. JB JS Open Access. 3:e0043. DOI: 10.2106/JBJS.OA.17.00043. PMID: 30229235. PMCID: PMC6132904.44. Hernigou P, Flouzat Lachaniette CH, Delambre J, Zilber S, Duffiet P, Chevallier N, Rouard H. 2014; Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: a case-controlled study. Int Orthop. 38:1811–1818. DOI: 10.1007/s00264-014-2391-1. PMID: 24913770.
Article45. Jo CH, Chai JW, Jeong EC, Oh S, Yoon KS. 2020; Intratendinous injection of mesenchymal stem cells for the treatment of rotator cuff disease: a 2-year follow-up study. Arthroscopy. 36:971–980. DOI: 10.1016/j.arthro.2019.11.120. PMID: 31805388.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Overviews on the Clinical Use of Stem Cells in Orthopaedics
- Autologous Stem Cell Application in Periodontal Regeneration Technique (SAI-PRT) Using PDLSCs Directly From an Extracted Tooth...An Insight
- Rotator Cuff Tendon Regeneration
- Current Progress in Tendon and Ligament Tissue Engineering
- Latest Trends in Non-Clinical and Clinical Research on Mesenchymal Stem Cell Therapy for Tendon Regeneration