Korean J Gastroenterol.
2020 Nov;76(5):232-237. 10.4166/kjg.2020.139.
Prevention of Non-steroidal Anti-inflammatory Drug-induced Peptic Ulcers
- Affiliations
-
- 1Division of Gastroenterology, Department of Internal Medicine, Incheon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2508871
- DOI: http://doi.org/10.4166/kjg.2020.139
Abstract
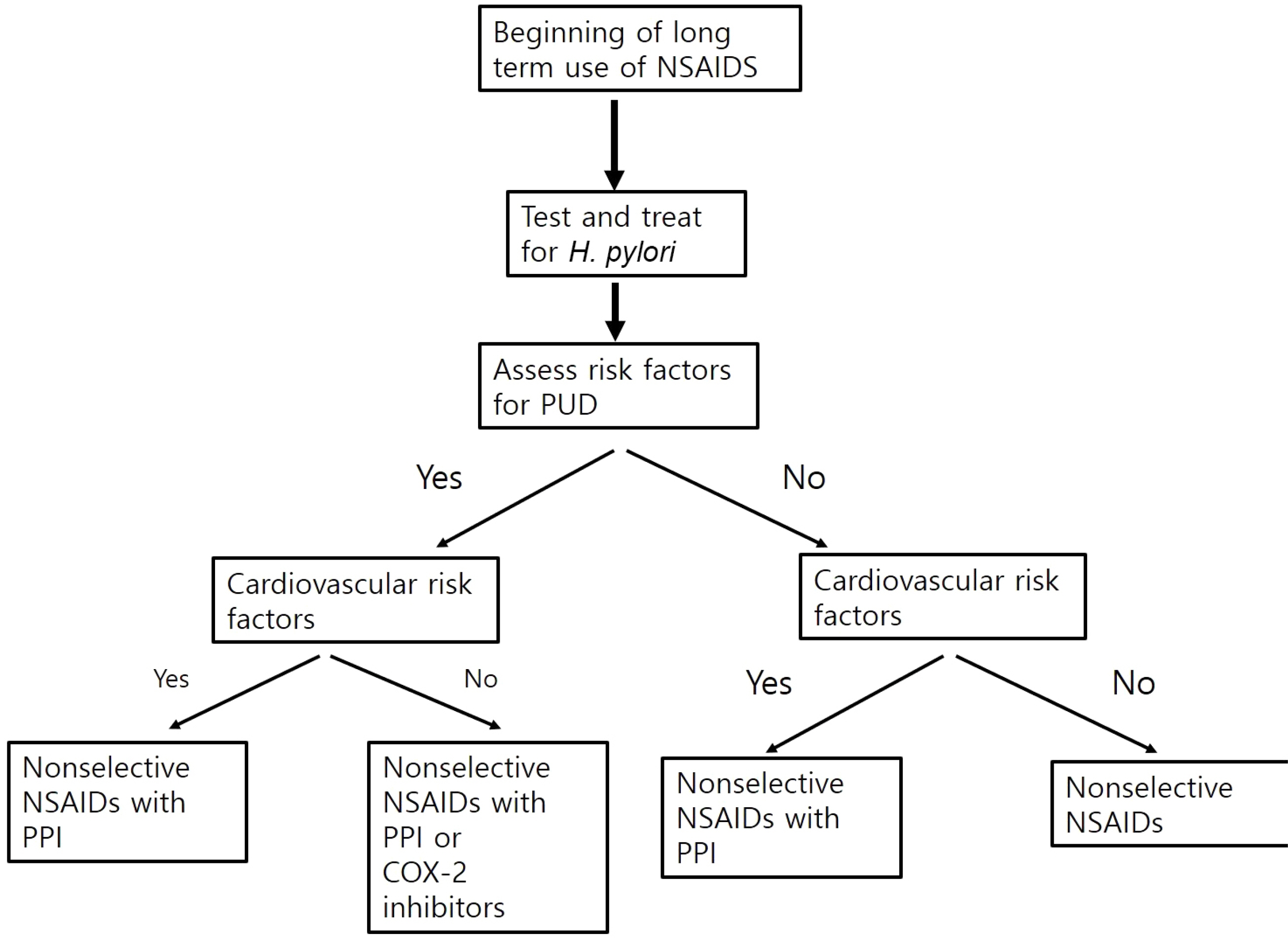
- Nonsteroidal anti-inflammatory drugs (NSAID) are some of the most commonly prescribed medications in clinical practice. The long-term use of NSAIDs is one of the main causes of peptic ulcers and the increased risk of upper gastrointestinal tract complications, such as perforation and bleeding. Thus, the prevention of NSAID-induced peptic ulcers is an important clinical issue. Previous studies have evaluated various strategies for preventing ulcers in patients requiring prolonged NSAID use. The Korean clinical practice guidelines have been published recently based on the evidence of the currently available data. This review describes the strategies for the prevention of peptic ulcers due to NSAID. An assessment of the risk factors for peptic ulcers from NSAID is recommended to identify patients who should be considered for primary prophylaxis. The risk of NSAID-induced peptic ulcers can be reduced by the concomitant use of proton pump inhibitors (PPI), misoprostol, and histamine-2 receptor antagonists. Selective cyclooxygenase-2 inhibitors can be used with caution due to concerns regarding cardiovascular toxicity. Attempts should be made to use the lowest dose and shortest duration of the NSAID.
Figure
Reference
-
1. Wallace JL. 1997; Nonsteroidal anti-inflammatory drugs and gastroenteropathy: the second hundred years. Gastroenterology. 112:1000–1016. DOI: 10.1053/gast.1997.v112.pm9041264. PMID: 9041264.
Article2. Abraham NS. 2014; Gastrointestinal bleeding in cardiac patients: epidemiology and evolving clinical paradigms. Curr Opin Gastroenterol. 30:609–614. DOI: 10.1097/MOG.0000000000000122. PMID: 25216111.3. Wongrakpanich S, Wongrakpanich A, Melhado K, Rangaswami J. 2018; A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging Dis. 9:143–150. DOI: 10.14336/AD.2017.0306. PMID: 29392089. PMCID: PMC5772852.
Article4. Shim YK, Kim N. 2016; Nonsteroidal anti-inflammatory drug and aspirin-induced peptic ulcer disease. Korean J Gastroenterol. 67:300–312. DOI: 10.4166/kjg.2016.67.6.300. PMID: 27312830.
Article5. Armstrong CP, Blower AL. 1987; Non-steroidal anti-inflammatory drugs and life threatening complications of peptic ulceration. Gut. 28:527–532. DOI: 10.1136/gut.28.5.527. PMID: 3596334. PMCID: PMC1432891.
Article6. Manuel D, Cutler A, Goldstein J, Fennerty MB, Brown K. 2007; Decreasing prevalence combined with increasing eradication of Helicobacter pylori infection in the United States has not resulted in fewer hospital admissions for peptic ulcer disease-related complications. Aliment Pharmacol Ther. 25:1423–1427. DOI: 10.1111/j.1365-2036.2007.03340.x. PMID: 17539981.
Article7. Post PN, Kuipers EJ, Meijer GA. 2006; Declining incidence of peptic ulcer but not of its complications: a nation-wide study in The Netherlands. Aliment Pharmacol Ther. 23:1587–1593. DOI: 10.1111/j.1365-2036.2006.02918.x. PMID: 16696807.
Article8. Bhatt DL, Scheiman J, Abraham NS, et al. 2008; ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 52:1502–1517. DOI: 10.1016/j.jacc.2008.08.002. PMID: 19017521.9. Lanza FL, Chan FK, Quigley EM. Practice Parameters Committee of the American College of Gastroenterology. 2009; Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol. 104:728–738. DOI: 10.1038/ajg.2009.115. PMID: 19240698.
Article10. Joo MK, Park CH, Kim JS, et al. 2020; Clinical guidelines for drug-induced peptic ulcer, 2020 revised edition. Korean J Gastroenterol. 76:108–133. DOI: 10.4166/kjg.2020.76.3.108. PMID: 32969360.
Article11. Laine L, Curtis SP, Cryer B, Kaur A, Cannon CP. 2010; Risk factors for NSAID-associated upper GI clinical events in a long-term prospective study of 34 701 arthritis patients. Aliment Pharmacol Ther. 32:1240–1248. DOI: 10.1111/j.1365-2036.2010.04465.x. PMID: 20955443.12. Lee HL, Han DS, Kim JB, et al. 2004; Importance of age and other risk factors in NSAID-induced gastropathy. Korean J Gastroenterol. 44:246–251. PMID: 15564803.13. Loke YK, Trivedi AN, Singh S. 2008; Meta-analysis: gastrointestinal bleeding due to interaction between selective serotonin uptake inhibitors and non-steroidal anti-inflammatory drugs. Aliment Pharmacol Ther. 27:31–40. DOI: 10.1111/j.1365-2036.2007.03541.x. PMID: 17919277.
Article14. Bhala N, Emberson J, et al. Coxib and traditional NSAID Trialists' (CNT) Collaboration. 2013; Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet. 382:769–779. DOI: 10.1016/S0140-6736(13)60900-9. PMID: 23726390. PMCID: PMC3778977.15. Richy F, Bruyere O, Ethgen O, et al. 2004; Time dependent risk of gastrointestinal complications induced by non-steroidal anti-inflammatory drug use: a consensus statement using a meta-analytic approach. Ann Rheum Dis. 63:759–766. DOI: 10.1136/ard.2003.015925. PMID: 15194568. PMCID: PMC1755051.
Article16. Henry D, Lim LL, Garcia Rodriguez LA, et al. 1996; Variability in risk of gastrointestinal complications with individual non-steroidal anti-inflammatory drugs: results of a collaborative meta-analysis. BMJ. 312:1563–1566. DOI: 10.1136/bmj.312.7046.1563. PMID: 8664664. PMCID: PMC2351326.
Article17. Papatheodoridis GV, Sougioultzis S, Archimandritis AJ. 2006; Effects of Helicobacter pylori and nonsteroidal anti-inflammatory drugs on peptic ulcer disease: a systematic review. Clin Gastroenterol Hepatol. 4:130–142. DOI: 10.1016/j.cgh.2005.10.006. PMID: 16469671.
Article18. Huang JQ, Sridhar S, Hunt RH. 2002; Role of Helicobacter pylori infection and non-steroidal anti-inflammatory drugs in peptic-ulcer disease: a meta-analysis. Lancet. 359:14–22. DOI: 10.1016/S0140-6736(02)07273-2. PMID: 11809181.19. Kiltz U, Zochling J, Schmidt WE, Braun J. 2008; Use of NSAIDs and infection with Helicobacter pylori--what does the rheumatologist need to know? Rheumatology (Oxford). 47:1342–1347. DOI: 10.1093/rheumatology/ken123. PMID: 18477642.
Article20. Chan FK, Sung JJ, Chung SC, et al. 1997; Randomised trial of eradication of Helicobacter pylori before non-steroidal anti-inflammatory drug therapy to prevent peptic ulcers. Lancet. 350:975–979. DOI: 10.1016/S0140-6736(97)04523-6. PMID: 9329511.
Article21. Chan FK, To KF, Wu JC, et al. 2002; Eradication of Helicobacter pylori and risk of peptic ulcers in patients starting long-term treatment with non-steroidal anti-inflammatory drugs: a randomised trial. Lancet. 359:9–13. DOI: 10.1016/S0140-6736(02)07272-0. PMID: 11809180.22. Labenz J, Blum AL, Bolten WW, et al. 2002; Primary prevention of diclofenac associated ulcers and dyspepsia by omeprazole or triple therapy in Helicobacter pylori positive patients: a randomised, double blind, placebo controlled, clinical trial. Gut. 51:329–335. DOI: 10.1136/gut.51.3.329. PMID: 12171952. PMCID: PMC1773346.
Article23. Hawkey CJ, Tulassay Z, Szczepanski L, et al. 1998; Randomised controlled trial of Helicobacter pylori eradication in patients on non-steroidal anti-inflammatory drugs: HELP NSAIDs study. Helicobacter eradication for lesion prevention. Lancet. 352:1016–1021. DOI: 10.1016/S0140-6736(98)04206-8. PMID: 9759744.24. Lai KC, Lau CS, Ip WY, et al. 2003; Effect of treatment of Helicobacter pylori on the prevention of gastroduodenal ulcers in patients receiving long-term NSAIDs: a double-blind, placebo-controlled trial. Aliment Pharmacol Ther. 17:799–805. DOI: 10.1046/j.1365-2036.2003.01528.x. PMID: 12641502.
Article25. de Leest HT, Steen KS, Lems WF, et al. 2007; Eradication of Helicobacter pylori does not reduce the incidence of gastroduodenal ulcers in patients on long-term NSAID treatment: double-blind, randomized, placebo-controlled trial. Helicobacter. 12:477–485. DOI: 10.1111/j.1523-5378.2007.00543.x. PMID: 17760715.
Article26. Song HJ, Kwon JW, Kim N, Park YS. 2013; Cost effectiveness associated with Helicobacter pylori screening and eradication in patients taking nonsteroidal anti-inflammatory drugs and/or aspirin. Gut Liver. 7:182–189. DOI: 10.5009/gnl.2013.7.2.182. PMID: 23560154. PMCID: PMC3607772.
Article27. Zidar N, Odar K, Glavac D, Jerse M, Zupanc T, Stajer D. 2009; Cyclooxygenase in normal human tissues--is COX-1 really a constitutive isoform, and COX-2 an inducible isoform? J Cell Mol Med. 13:3753–3763. DOI: 10.1111/j.1582-4934.2008.00430.x. PMID: 18657230. PMCID: PMC4516524.28. Feldman M, McMahon AT. 2000; Do cyclooxygenase-2 inhibitors provide benefits similar to those of traditional nonsteroidal anti-inflammatory drugs, with less gastrointestinal toxicity? Ann Intern Med. 132:134–143. DOI: 10.7326/0003-4819-132-2-200001180-00008. PMID: 10644275.
Article29. Goldstein JL, Cryer B, Amer F, Hunt B. 2007; Celecoxib plus aspirin versus naproxen and lansoprazole plus aspirin: a randomized, double-blind, endoscopic trial. Clin Gastroenterol Hepatol. 5:1167–1174. DOI: 10.1016/j.cgh.2007.06.009. PMID: 17916545.
Article30. Laine L, Curtis SP, Cryer B, Kaur A, Cannon CP. MEDAL Steering Committee. 2007; Assessment of upper gastrointestinal safety of etoricoxib and diclofenac in patients with osteoarthritis and rheumatoid arthritis in the multinational etoricoxib and diclofenac arthritis long-term (MEDAL) programme: a randomised comparison. Lancet. 369:465–473. DOI: 10.1016/S0140-6736(07)60234-7. PMID: 17292766.
Article31. Targownik LE, Metge CJ, Leung S, Chateau DG. 2008; The relative efficacies of gastroprotective strategies in chronic users of nonsteroidal anti-inflammatory drugs. Gastroenterology. 134:937–944. DOI: 10.1053/j.gastro.2008.01.010. PMID: 18294634.
Article32. Farkouh ME, Greenberg BP. 2009; An evidence-based review of the cardiovascular risks of nonsteroidal anti-inflammatory drugs. Am J Cardiol. 103:1227–1237. DOI: 10.1016/j.amjcard.2009.01.014. PMID: 19406264.
Article33. Bhatt DL, Scheiman J, Abraham NS, et al. 2008; ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on clinical expert consensus documents. Circulation. 118:1894–1909. DOI: 10.1161/CIRCULATIONAHA.108.191087. PMID: 18836135.34. Satoh K, Yoshino J, Akamatsu T, et al. 2016; Evidence-based clinical practice guidelines for peptic ulcer disease 2015. J Gastroenterol. 51:177–194. DOI: 10.1007/s00535-016-1166-4. PMID: 26879862.
Article35. Fallone CA, Chiba N, van Zanten SV, et al. 2016; The Toronto consensus for the treatment of Helicobacter pylori infection in adults. Gastroenterology. 151:51–69.e14. DOI: 10.1053/j.gastro.2016.04.006. PMID: 27102658.
Article36. Yeomans ND, Tulassay Z, Juhász L, et al. 1998; A comparison of omeprazole with ranitidine for ulcers associated with nonsteroidal antiinflammatory drugs. Acid suppression trial: ranitidine versus omeprazole for NSAID-associated ulcer treatment (ASTRONAUT) study group. N Engl J Med. 338:719–726. DOI: 10.1056/NEJM199803123381104. PMID: 9494148.37. Agrawal NM, Campbell DR, Safdi MA, Lukasik NL, Huang B, Haber MM. 2000; Superiority of lansoprazole vs ranitidine in healing nonsteroidal anti-inflammatory drug-associated gastric ulcers: results of a double-blind, randomized, multicenter study. NSAIDassociated gastric ulcer study group. Arch Intern Med. 160:1455–1461. DOI: 10.1001/archinte.160.10.1455. PMID: 10826458.38. Hawkey CJ, Karrasch JA, Szczepañski L, et al. 1998; Omeprazole compared with misoprostol for ulcers associated with nonsteroidal antiinflammatory drugs. Omeprazole versus misoprostol for NSAID-induced ulcer management (OMNIUM) Study Group. N Engl J Med. 338:727–734. DOI: 10.1056/NEJM199803123381105. PMID: 9494149.39. Bianchi Porro G, Lazzaroni M, Manzionna G, Petrillo M. 1998; Omeprazole and sucralfate in the treatment of NSAID-induced gastric and duodenal ulcer. Aliment Pharmacol Ther. 12:355–360. DOI: 10.1046/j.1365-2036.1998.00312.x. PMID: 9690725.40. Kim JJ, Kim N, Lee BH, et al. 2010; Risk factors for development and recurrence of peptic ulcer disease. Korean J Gastroenterol. 56:220–228. DOI: 10.4166/kjg.2010.56.4.220. PMID: 20962557.
Article41. Scheiman JM, Yeomans ND, Talley NJ, et al. 2006; Prevention of ulcers by esomeprazole in at-risk patients using non-selective NSAIDs and COX-2 inhibitors. Am J Gastroenterol. 101:701–710. DOI: 10.1111/j.1572-0241.2006.00499.x. PMID: 16494585.
Article42. Yeomans ND, Graham DY, Husni ME, et al. 2018; Randomised clinical trial: gastrointestinal events in arthritis patients treated with celecoxib, ibuprofen or naproxen in the PRECISION trial. Aliment Pharmacol Ther. 47:1453–1463. DOI: 10.1111/apt.14610. PMID: 29667211.
Article43. Raskin JB, White RH, Jackson JE, et al. 1995; Misoprostol dosage in the prevention of nonsteroidal anti-inflammatory drug-induced gastric and duodenal ulcers: a comparison of three regimens. Ann Intern Med. 123:344–350. DOI: 10.7326/0003-4819-123-5-199509010-00004. PMID: 7625622.
Article44. Silverstein FE, Graham DY, Senior JR, et al. 1995; Misoprostol reduces serious gastrointestinal complications in patients with rheumatoid arthritis receiving nonsteroidal anti-inflammatory drugs. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 123:241–249. DOI: 10.7326/0003-4819-123-4-199508150-00001. PMID: 7611589.45. Graham DY, Agrawal NM, Campbell DR, et al. 2002; Ulcer prevention in long-term users of nonsteroidal anti-inflammatory drugs: results of a double-blind, randomized, multicenter, active- and placebo-controlled study of misoprostol vs lansoprazole. Arch Intern Med. 162:169–175. DOI: 10.1001/archinte.162.2.169. PMID: 11802750.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diagnosis and treatment of Helicobacter pylori infection in patients treated with non-steroidal anti-inflammatory drugs
- Guidelines of Prevention and Treatment for NSAID-related Peptic Ulcers
- Update on NSAIDs Related Peptic Ulcers
- Multiple Gastrointestinal Drug Induced Ulcers Associated with Aspirin and Non-steroidal Anti-inflammatory Drugs: A Case Report and Review of the Literature
- Non-steroidal anti-inflammatory drug-induced enteropathy