Korean J Physiol Pharmacol.
2020 Nov;24(6):517-527. 10.4196/kjpp.2020.24.6.517.
Layer-specific serotonergic induction of long-term depression in the prefrontal cortex of rats
- Affiliations
-
- 1Department of Physiology, College of Medicine, The Catholic University of Korea, Seoul 06591, Korea
- 2Department of Catholic Neuroscience Institute, College of Medicine, The Catholic University of Korea, Seoul 06591, Korea
- KMID: 2507734
- DOI: http://doi.org/10.4196/kjpp.2020.24.6.517
Abstract
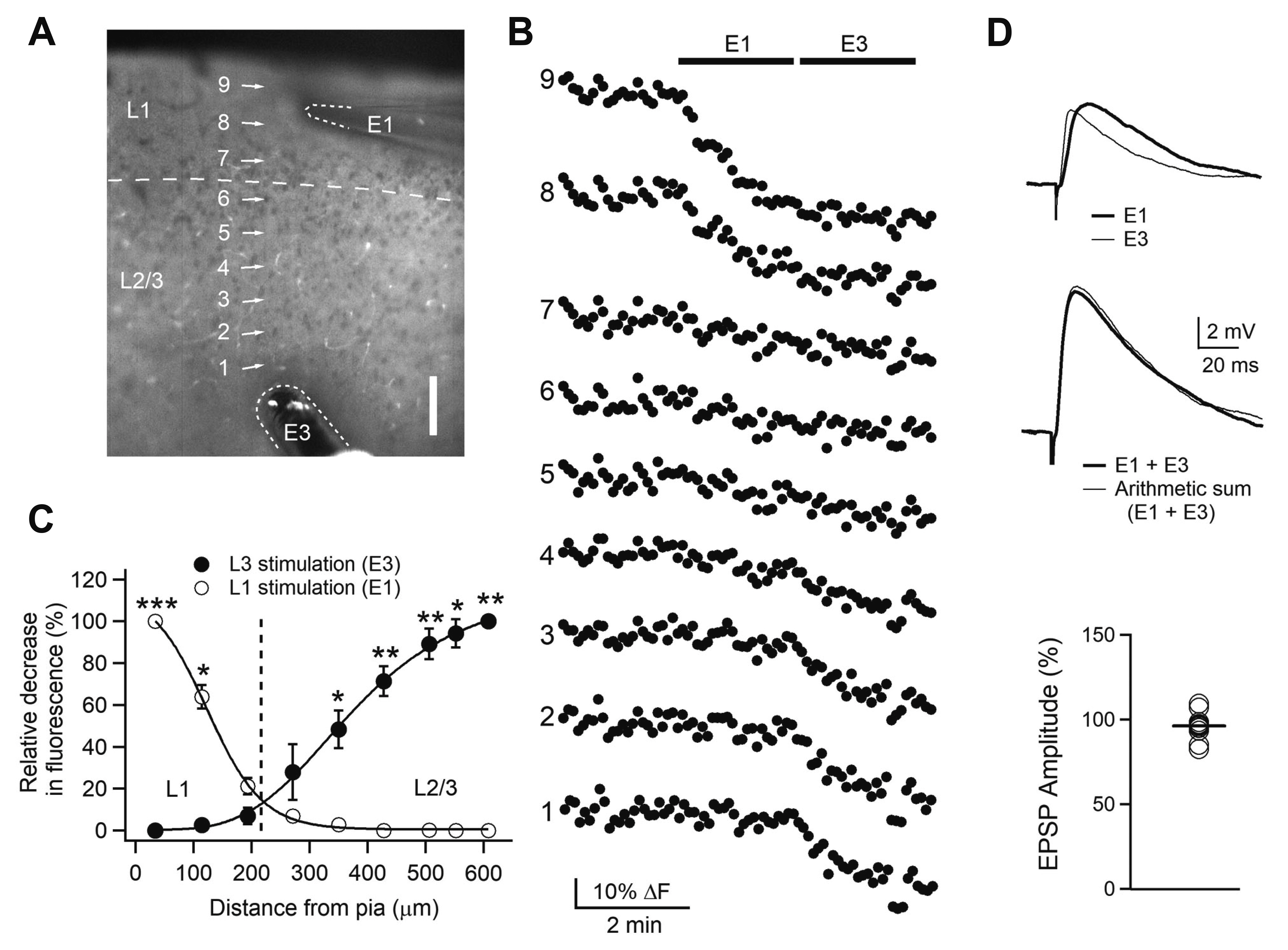
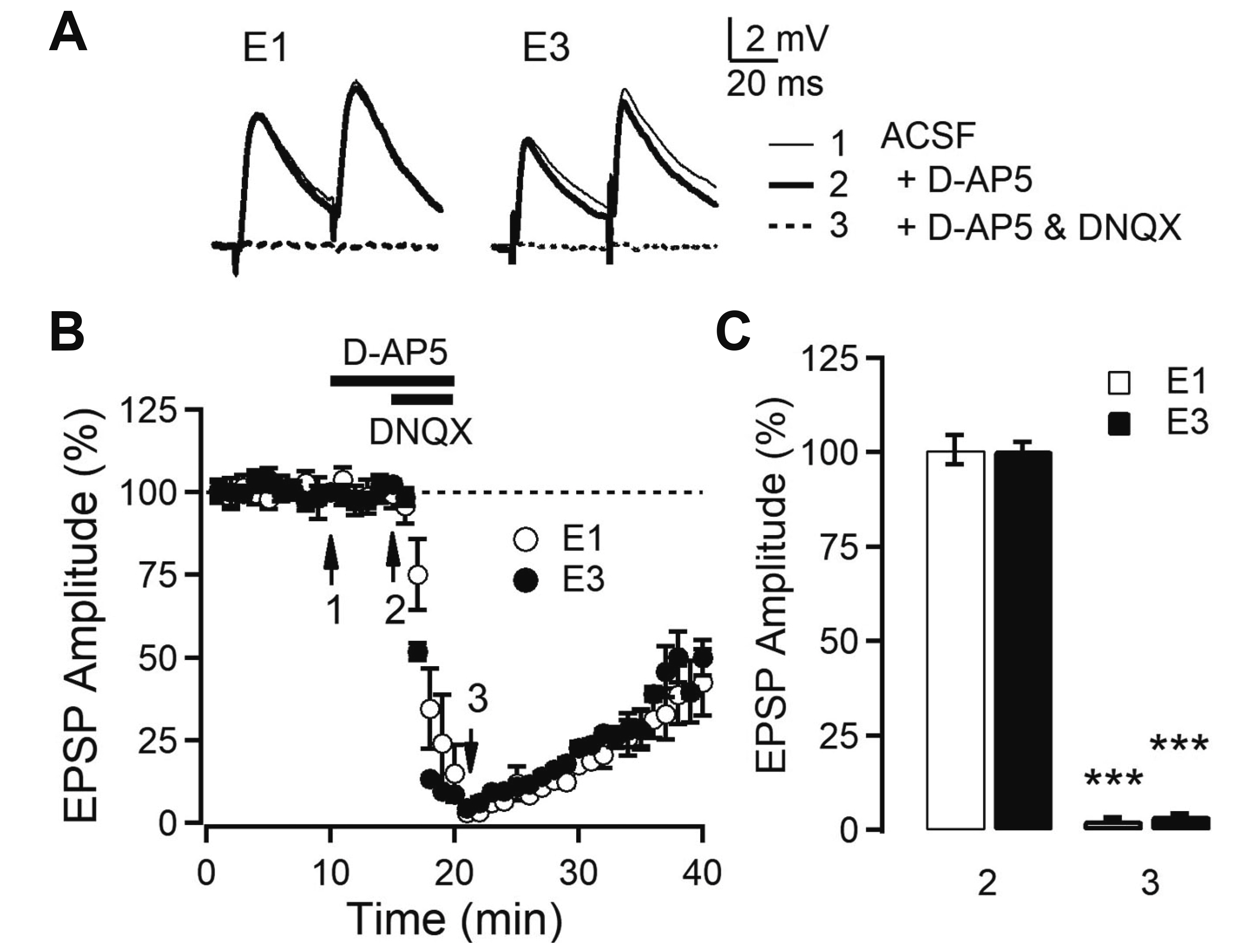
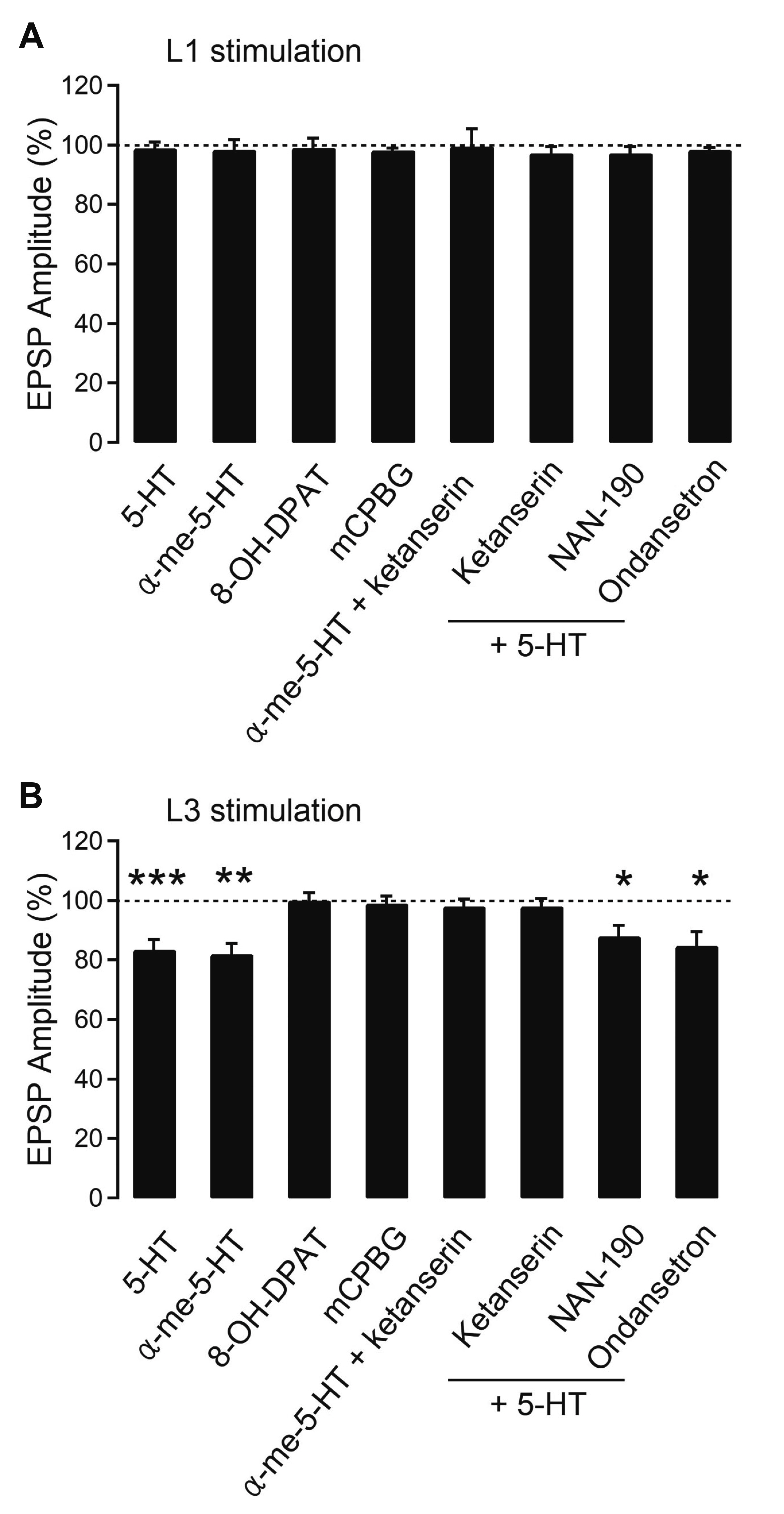
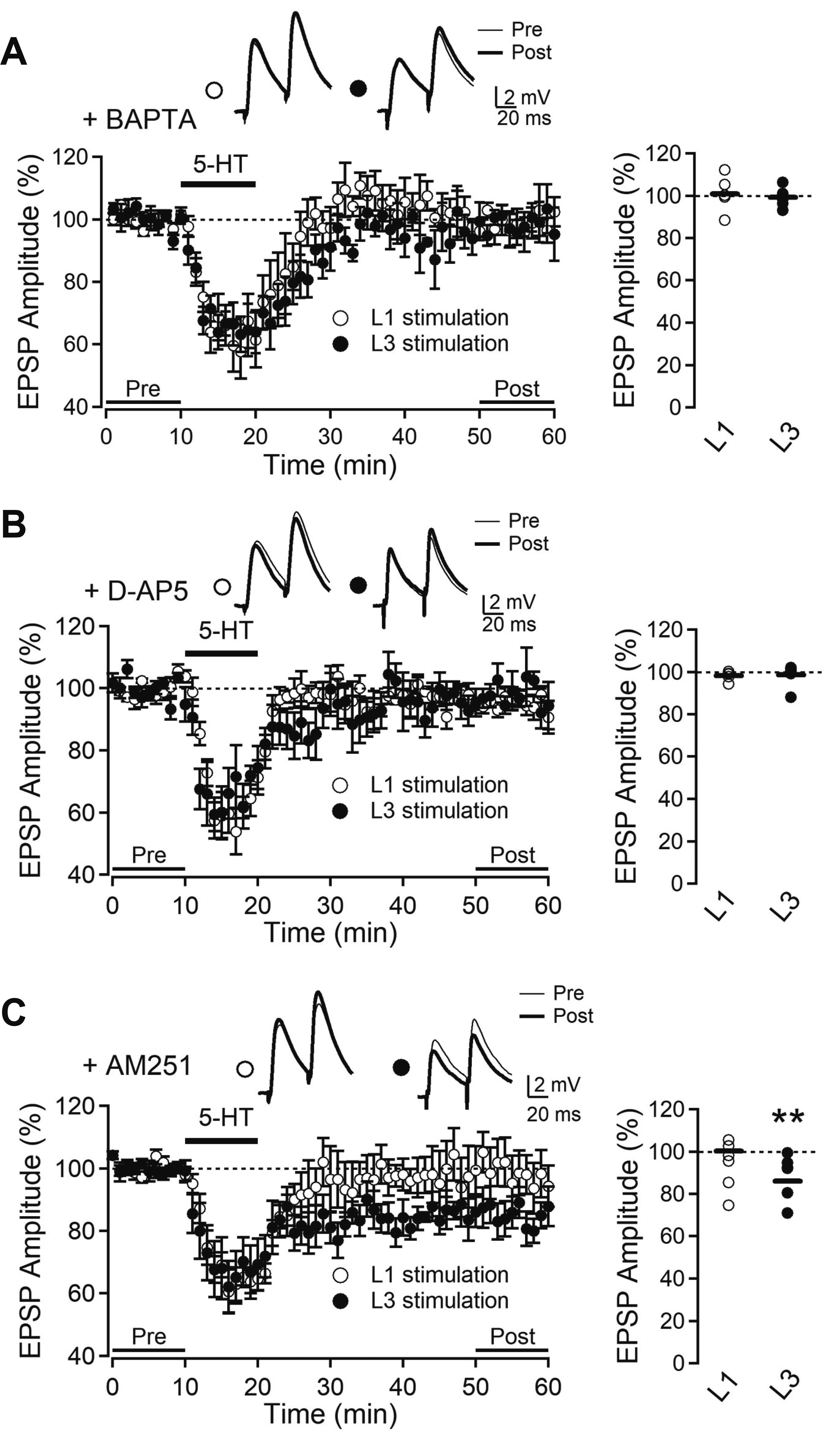
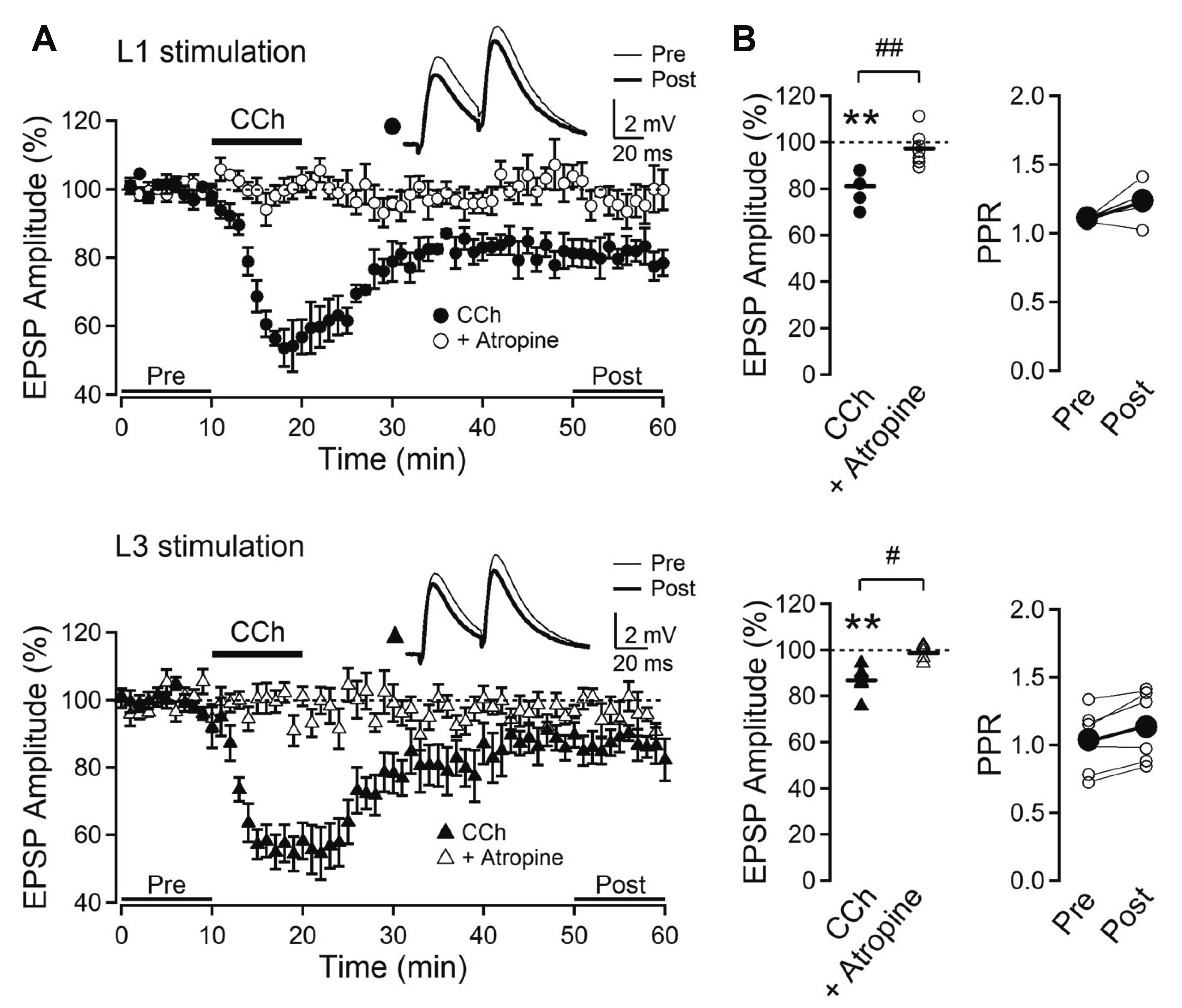
- Layer 2/3 pyramidal neurons (L2/3 PyNs) of the cortex extend their basal dendrites near the soma and as apical dendritic tufts in layer 1, which mainly receive feedforward and feedback inputs, respectively. It is suggested that neuromodulators such as serotonin and acetylcholine may regulate the information flow between brain structures depending on the brain state. However, little is known about the dendritic compartment-specific induction of synaptic transmission in single PyNs. Here, we studied layer-specific serotonergic and cholinergic induction of long-term synaptic plasticity in L2/3 PyNs of the agranular insular cortex, a lateral component of the orbitofrontal cortex. Using FM1-43 dye unloading, we verified that local electrical stimulation to layers 1 (L1) and 3 (L3) activated axon terminals mostly located in L1 and perisomatic area (L2/3). Independent and AMPA receptor-mediated excitatory postsynaptic potential was evoked by local electrical stimulation of either L1 or L3. Application of serotonin (5-HT, 10 μM) induced activity-dependent longterm depression (LTD) in L2/3 but not in L1 inputs. LTD induced by 5-HT was blocked by the 5-HT2 receptor antagonist ketanserin, an NMDA receptor antagonist and by intracellular Ca2+ chelation. The 5-HT2 receptor agonist α-me-5-HT mimicked the LTD induced by 5-HT. However, the application of carbachol induced muscarinic receptor-dependent LTD in both inputs. The differential layer-specific induction of LTD by neuromodulators might play an important role in information processing mechanism of the prefrontal cortex.
Figure
Reference
-
1. Larkum ME, Petro LS, Sachdev RNS, Muckli L. 2018; A perspective on cortical layering and layer-spanning neuronal elements. Front Neuroanat. 12:56. DOI: 10.3389/fnana.2018.00056. PMID: 30065634. PMCID: PMC6056619.
Article2. Larkman AU. 1991; Dendritic morphology of pyramidal neurones of the visual cortex of the rat: III. Spine distributions. J Comp Neurol. 306:332–343. DOI: 10.1002/cne.903060209. PMID: 1711059.
Article3. Sjöström PJ, Rancz EA, Roth A, Häusser M. 2008; Dendritic excitability and synaptic plasticity. Physiol Rev. 88:769–840. DOI: 10.1152/physrev.00016.2007. PMID: 18391179.
Article4. Marder E, O'Leary T, Shruti S. 2014; Neuromodulation of circuits with variable parameters: single neurons and small circuits reveal principles of state-dependent and robust neuromodulation. Annu Rev Neurosci. 37:329–346. DOI: 10.1146/annurev-neuro-071013-013958. PMID: 25032499.
Article5. Celada P, Puig MV, Artigas F. 2013; Serotonin modulation of cortical neurons and networks. Front Integr Neurosci. 7:25. DOI: 10.3389/fnint.2013.00025. PMID: 23626526. PMCID: PMC3630391.
Article6. Hasselmo ME, Sarter M. 2011; Modes and models of forebrain cholinergic neuromodulation of cognition. Neuropsychopharmacology. 36:52–73. DOI: 10.1038/npp.2010.104. PMID: 20668433. PMCID: PMC2992803.
Article7. Cho KH, Jang HJ, Jo YH, Singer W, Rhie DJ. 2012; Cholinergic induction of input-specific late-phase LTP via localized Ca2+ release in the visual cortex. J Neurosci. 32:4520–4530. DOI: 10.1523/JNEUROSCI.4577-11.2012. PMID: 22457499. PMCID: PMC6622052.8. Joo K, Cho KH, Youn SH, Jang HJ, Rhie DJ. 2019; Layer-specific involvement of endocannabinoid signaling in muscarinic-induced long-term depression in layer 2/3 pyramidal neurons of rat visual cortex. Brain Res. 1712:124–131. DOI: 10.1016/j.brainres.2019.02.007. PMID: 30753818.
Article9. Jang HJ, Cho KH, Park SW, Kim MJ, Yoon SH, Rhie DJ. 2012; Layer-specific serotonergic facilitation of IPSC in layer 2/3 pyramidal neurons of the visual cortex. J Neurophysiol. 107:407–416. DOI: 10.1152/jn.00535.2011. PMID: 22013240.
Article10. Puig MV, Gulledge AT. 2011; Serotonin and prefrontal cortex function: neurons, networks, and circuits. Mol Neurobiol. 44:449–464. DOI: 10.1007/s12035-011-8214-0. PMID: 22076606. PMCID: PMC3282112.
Article11. Miller EK, Cohen JD. 2001; An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 24:167–202. DOI: 10.1146/annurev.neuro.24.1.167. PMID: 11283309.
Article12. Ongür D, Price JL. 2000; The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 10:206–219. DOI: 10.1093/cercor/10.3.206. PMID: 10731217.13. Dalley JW, Cardinal RN, Robbins TW. 2004; Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. 28:771–784. DOI: 10.1016/j.neubiorev.2004.09.006. PMID: 15555683.
Article14. Izquierdo A. 2017; Functional heterogeneity within rat orbitofrontal cortex in reward learning and decision making. J Neurosci. 37:10529–10540. DOI: 10.1523/JNEUROSCI.1678-17.2017. PMID: 29093055. PMCID: PMC6596524.
Article15. Wallis JD. 2011; Cross-species studies of orbitofrontal cortex and value-based decision-making. Nat Neurosci. 15:13–19. DOI: 10.1038/nn.2956. PMID: 22101646. PMCID: PMC3549638.
Article16. Sul JH, Kim H, Huh N, Lee D, Jung MW. 2010; Distinct roles of rodent orbitofrontal and medial prefrontal cortex in decision making. Neuron. 66:449–460. DOI: 10.1016/j.neuron.2010.03.033. PMID: 20471357. PMCID: PMC2872629.
Article17. Gerfen CR, Clavier RM. 1979; Neural inputs to the prefrontal agranular insular cortex in the rat: horseradish peroxidase study. Brain Res Bull. 4:347–353. DOI: 10.1016/S0361-9230(79)80012-X. PMID: 90546.
Article18. Gabbott PL, Warner TA, Jays PR, Bacon SJ. 2003; Areal and synaptic interconnectivity of prelimbic (area 32), infralimbic (area 25) and insular cortices in the rat. Brain Res. 993:59–71. DOI: 10.1016/j.brainres.2003.08.056. PMID: 14642831.
Article19. Cruikshank SJ, Ahmed OJ, Stevens TR, Patrick SL, Gonzalez AN, Elmaleh M, Connors BW. 2012; Thalamic control of layer 1 circuits in prefrontal cortex. J Neurosci. 32:17813–17823. DOI: 10.1523/JNEUROSCI.3231-12.2012. PMID: 23223300. PMCID: PMC3535493.
Article20. Aggleton JP, Wright NF, Rosene DL, Saunders RC. 2015; Complementary patterns of direct amygdala and hippocampal projections to the macaque prefrontal cortex. Cereb Cortex. 25:4351–4373. DOI: 10.1093/cercor/bhv019. PMID: 25715284. PMCID: PMC4612443.
Article21. Kuramoto E, Iwai H, Yamanaka A, Ohno S, Seki H, Tanaka YR, Furuta T, Hioki H, Goto T. 2017; Dorsal and ventral parts of thalamic nucleus submedius project to different areas of rat orbitofrontal cortex: a single neuron-tracing study using virus vectors. J Comp Neurol. 525:3821–3839. DOI: 10.1002/cne.24306. PMID: 28863230.
Article22. Woolf NJ, Butcher LL. 2011; Cholinergic systems mediate action from movement to higher consciousness. Behav Brain Res. 221:488–498. DOI: 10.1016/j.bbr.2009.12.046. PMID: 20060422.
Article23. Smiley JF, Goldman-Rakic PS. 1996; Serotonergic axons in monkey prefrontal cerebral cortex synapse predominantly on interneurons as demonstrated by serial section electron microscopy. J Comp Neurol. 367:431–443. DOI: 10.1002/(SICI)1096-9861(19960408)367:3<431::AID-CNE8>3.0.CO;2-6. PMID: 8698902.
Article24. Kirkwood A. 2000; Serotonergic control of developmental plasticity. Proc Natl Acad Sci U S A. 97:1951–1952. DOI: 10.1073/pnas.070044697. PMID: 10688919. PMCID: PMC33510.
Article25. Zhong P, Liu W, Gu Z, Yan Z. 2008; Serotonin facilitates long-term depression induction in prefrontal cortex via p38 MAPK/Rab5-mediated enhancement of AMPA receptor internalization. J Physiol. 586:4465–4479. DOI: 10.1113/jphysiol.2008.155143. PMID: 18653660. PMCID: PMC2614015.26. Martin HG, Bernabeu A, Lassalle O, Bouille C, Beurrier C, Pelissier-Alicot AL, Manzoni OJ. 2015; Endocannabinoids mediate muscarinic acetylcholine receptor-dependent long-term depression in the adult medial prefrontal cortex. Front Cell Neurosci. 9:457. DOI: 10.3389/fncel.2015.00457. PMID: 26648844. PMCID: PMC4664641.
Article27. Huang CC, Hsu KS. 2010; Activation of muscarinic acetylcholine receptors induces a nitric oxide-dependent long-term depression in rat medial prefrontal cortex. Cereb Cortex. 20:982–996. DOI: 10.1093/cercor/bhp161. PMID: 19666830.
Article28. Ohashi S, Matsumoto M, Togashi H, Ueno K, Yoshioka M. 2003; The serotonergic modulation of synaptic plasticity in the rat hippocampo-medial prefrontal cortex pathway. Neurosci Lett. 342:179–182. DOI: 10.1016/S0304-3940(03)00293-3. PMID: 12757894.
Article29. Paxinos G, Watson C. 2007. The rat brain in stereotaxic coordinates. 6th ed. Academic Press/Elsevier;Amsterdam:30. Kay AR, Alfonso A, Alford S, Cline HT, Holgado AM, Sakmann B, Snitsarev VA, Stricker TP, Takahashi M, Wu LG. 1999; Imaging synaptic activity in intact brain and slices with FM1-43 in C. elegans, lamprey, and rat. Neuron. 24:809–817. DOI: 10.1016/S0896-6273(00)81029-6. PMID: 10624945.
Article31. Kirkwood A, Rozas C, Kirkwood J, Perez F, Bear MF. 1999; Modulation of long-term synaptic depression in visual cortex by acetylcholine and norepinephrine. J Neurosci. 19:1599–1609. DOI: 10.1523/JNEUROSCI.19-05-01599.1999. PMID: 10024347. PMCID: PMC6782177.
Article32. Shipp S. 2007; Structure and function of the cerebral cortex. Curr Biol. 17:R443–R449. DOI: 10.1016/j.cub.2007.03.044. PMID: 17580069.
Article33. Spruston N. 2008; Pyramidal neurons: dendritic structure and synaptic integration. Nat Rev Neurosci. 9:206–221. DOI: 10.1038/nrn2286. PMID: 18270515.
Article34. Berthoux C, Barre A, Bockaert J, Marin P, Bécamel C. 2019; Sustained activation of postsynaptic 5-HT2A receptors gates plasticity at prefrontal cortex synapses. Cereb Cortex. 29:1659–1669. DOI: 10.1093/cercor/bhy064. PMID: 29917056.35. Kim HS, Jang HJ, Cho KH, Hahn SJ, Kim MJ, Yoon SH, Jo YH, Kim MS, Rhie DJ. 2006; Serotonin inhibits the induction of NMDA receptor-dependent long-term potentiation in the rat primary visual cortex. Brain Res. 1103:49–55. DOI: 10.1016/j.brainres.2006.05.046. PMID: 16784733.
Article36. Jang HJ, Cho KH, Park SW, Kim MJ, Yoon SH, Rhie DJ. 2010; Effects of serotonin on the induction of long-term depression in the rat visual cortex. Korean J Physiol Pharmacol. 14:337–343. DOI: 10.4196/kjpp.2010.14.5.337. PMID: 21165334. PMCID: PMC2997421.
Article37. Park SW, Jang HJ, Cho KH, Kim MJ, Yoon SH, Rhie DJ. 2012; Developmental switch of the serotonergic role in the induction of synaptic long-term potentiation in the rat visual cortex. Korean J Physiol Pharmacol. 16:65–70. DOI: 10.4196/kjpp.2012.16.1.65. PMID: 22416222. PMCID: PMC3298828.
Article38. Kojic L, Dyck RH, Gu Q, Douglas RM, Matsubara J, Cynader MS. 2000; Columnar distribution of serotonin-dependent plasticity within kitten striate cortex. Proc Natl Acad Sci U S A. 97:1841–1844. DOI: 10.1073/pnas.97.4.1841. PMID: 10677543. PMCID: PMC26523.
Article39. Kojic L, Gu Q, Douglas RM, Cynader MS. 1997; Serotonin facilitates synaptic plasticity in kitten visual cortex: an in vitro study. Brain Res Dev Brain Res. 101:299–304. DOI: 10.1016/S0165-3806(97)00083-7. PMID: 9263606.40. Cornea-Hébert V, Riad M, Wu C, Singh SK, Descarries L. 1999; Cellular and subcellular distribution of the serotonin 5-HT2A receptor in the central nervous system of adult rat. J Comp Neurol. 409:187–209. DOI: 10.1002/(SICI)1096-9861(19990628)409:2<187::AID-CNE2>3.0.CO;2-P. PMID: 10379914.41. Clemett DA, Punhani T, Duxon MS, Blackburn TP, Fone KC. 2000; Immunohistochemical localisation of the 5-HT2C receptor protein in the rat CNS. Neuropharmacology. 39:123–132. DOI: 10.1016/S0028-3908(99)00086-6. PMID: 10665825.42. Li QH, Nakadate K, Tanaka-Nakadate S, Nakatsuka D, Cui Y, Watanabe Y. 2004; Unique expression patterns of 5-HT2A and 5-HT2C receptors in the rat brain during postnatal development: Western blot and immunohistochemical analyses. J Comp Neurol. 469:128–140. DOI: 10.1002/cne.11004. PMID: 14689478.43. Barre A, Berthoux C, De Bundel D, Valjent E, Bockaert J, Marin P, Bécamel C. 2016; Presynaptic serotonin 2A receptors modulate thalamocortical plasticity and associative learning. Proc Natl Acad Sci U S A. 113:E1382–E1391. DOI: 10.1073/pnas.1525586113. PMID: 26903620. PMCID: PMC4791007.
Article44. Otmakhova NA, Otmakhov N, Lisman JE. 2002; Pathway-specific properties of AMPA and NMDA-mediated transmission in CA1 hippocampal pyramidal cells. J Neurosci. 22:1199–1207. DOI: 10.1523/JNEUROSCI.22-04-01199.2002. PMID: 11850447. PMCID: PMC6757557.
Article45. Acker CD, Hoyos E, Loew LM. 2016; EPSPs measured in proximal dendritic spines of cortical pyramidal neurons. eNeuro. 3:ENEURO.0050-15.2016. DOI: 10.1523/ENEURO.0050-15.2016. PMID: 27257618. PMCID: PMC4874537.
Article46. Williams SR, Stuart GJ. 2002; Dependence of EPSP efficacy on synapse location in neocortical pyramidal neurons. Science. 295:1907–1910. DOI: 10.1126/science.1067903. PMID: 11884759.
Article47. Rao Y, Daw NW. 2004; Layer variations of long-term depression in rat visual cortex. J Neurophysiol. 92:2652–2658. DOI: 10.1152/jn.00298.2004. PMID: 15212419.
Article48. Markram H, Segal M. 1990; Long-lasting facilitation of excitatory postsynaptic potentials in the rat hippocampus by acetylcholine. J Physiol. 427:381–393. DOI: 10.1113/jphysiol.1990.sp018177. PMID: 2145426. PMCID: PMC1189936.
Article49. Picciotto MR, Higley MJ, Mineur YS. 2012; Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron. 76:116–129. DOI: 10.1016/j.neuron.2012.08.036. PMID: 23040810. PMCID: PMC3466476.
Article50. Hasselmo ME, McGaughy J. 2004; High acetylcholine levels set circuit dynamics for attention and encoding and low acetylcholine levels set dynamics for consolidation. Prog Brain Res. 145:207–231. DOI: 10.1016/S0079-6123(03)45015-2. PMID: 14650918.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Fluoxetine on the Induction of Long-term Potentiation in Rat Frontal Cortex
- Developmental Switch of the Serotonergic Role in the Induction of Synaptic Long-term Potentiation in the Rat Visual Cortex
- Effects of Serotonin on the Induction of Long-term Depression in the Rat Visual Cortex
- The Three Musketeers in the Medial Prefrontal Cortex: Subregion-specific Structural and Functional Plasticity Underlying Fear Memory Stages
- Global Cerebral Ischemia-induced Depression Accompanies Alteration of Neuronal Excitability in the Infralimbic Cortex Layer 2/3 Pyramidal Neurons