Korean Circ J.
2020 Sep;50(9):804-816. 10.4070/kcj.2020.0055.
Effects of Long-term Thrombin Inhibition (Dabigatran Etexilate) on Spontaneous Thrombolytic Activity during the Progression of Atherosclerosis in ApoE−/−–LDLR−/− Double-Knockout Mice
- Affiliations
-
- 1Laboratory of Medical Technology, Faculty of Nutrition, Kobe Gakuin University, Kobe, Japan
- 2Medical Corporation, Jinkeikai Ishii Hospital, Akashi, Japan
- KMID: 2505736
- DOI: http://doi.org/10.4070/kcj.2020.0055
Abstract
- Background and Objectives
Atherosclerosis is characterized by a hypercoagulable state, during which coagulation and fibrinolytic factors are activated simultaneously. However, details regarding the thrombolytic pathway in this context remain unknown. Here we investigated how direct long-term inhibition of thrombin influenced spontaneous thrombolytic activity during atherosclerotic progression in apolipoprotein E (ApoE)–/––low density lipoprotein receptor (LDLR)–/– double-knockout mice.
Methods
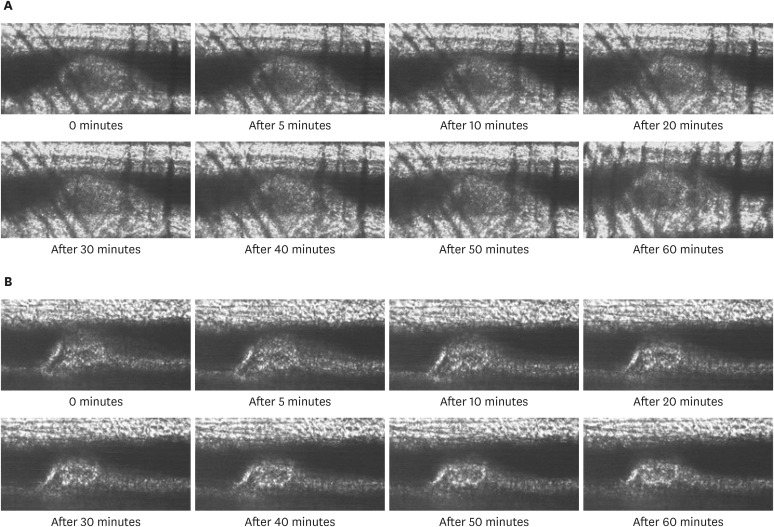
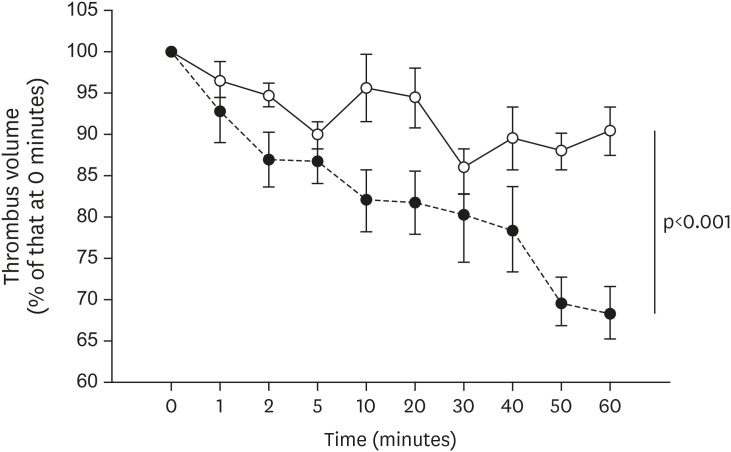
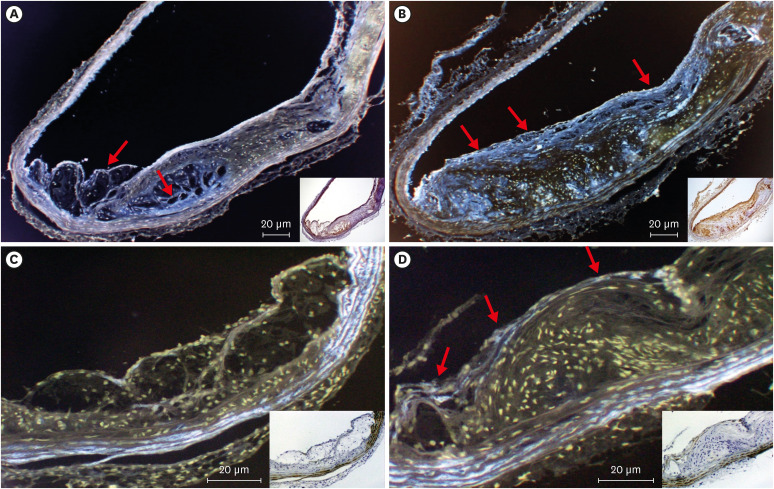
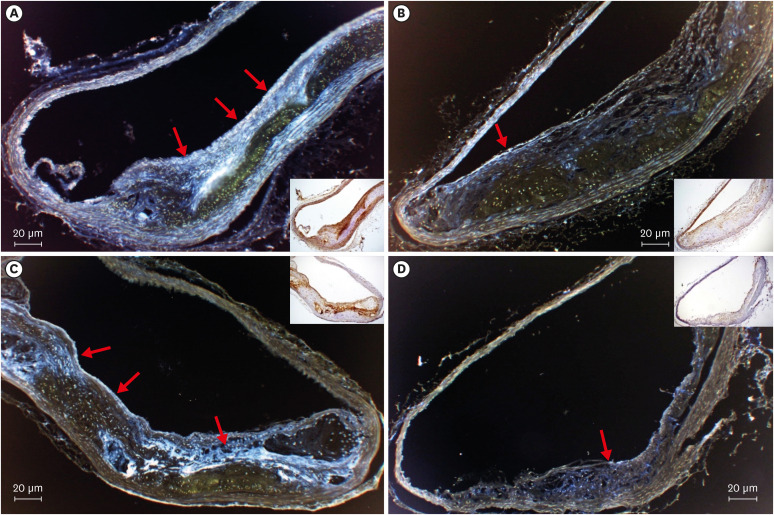
All mice received either standard chow (placebo group) or dabigatran-containing chow for 22 weeks, after which we evaluated them. The amount of atherosclerosis was estimated as the ratio of the atherosclerotic area to the total aortic intimal area. In addition, we used immunohistochemistry to analyze the expression of tissue plasminogen activator (t-PA), plasminogen activator inhibitor-1 (PAI-1), thrombin activatable fibrinolysis inhibitor (TAFI), and endothelial nitric oxide synthase (eNOS) in atherosclerotic regions. To evaluate thrombolysis, we used a He–Ne laser to induce thrombosis in vessels of the cremaster muscle and then measured the thrombus volume over time.
Results
The atherosclerotic area was smaller and thrombolytic activity greater in the dabigatran-treated group than in the placebo group. Furthermore, according to the thrombolysis model, spontaneous thrombolytic activity was increased in the dabigatran-treated mice compared with the placebo mice. In support of these results, immunohistochemistry demonstrated decreased expression of PAI-1 and TAFI but increased expression of eNOS in the dabigatran group compared with the placebo group. However, t-PA expression did not differ between groups.
Conclusions
Direct long-term inhibition by dabigatran etexilate of thrombin led to an increase in spontaneous thrombolytic activity decreasing the expression of PAI-1 and TAFI.
Keyword
Figure
Reference
-
1. Lippi G, Franchini M, Targher G. Arterial thrombus formation in cardiovascular disease. Nat Rev Cardiol. 2011; 8:502–512. PMID: 21727917.
Article2. Davies MJ. Stability and instability: two faces of coronary atherosclerosis. The Paul Dudley White Lecture 1995. Circulation. 1996; 94:2013–2020. PMID: 8873680.3. Esmon CT. Targeting factor Xa and thrombin: impact on coagulation and beyond. Thromb Haemost. 2014; 111:625–633. PMID: 24336942.
Article4. Adams HP Jr, Brott TG, Furlan AJ, et al. Guidelines for thrombolytic therapy for acute stroke: a supplement to the guidelines for the management of patients with acute ischemic stroke. A statement for healthcare professionals from a Special Writing Group of the Stroke Council, American Heart Association. Stroke. 1996; 27:1711–1718. PMID: 8784157.5. Hankey GJ, Eikelboom JW. Dabigatran etexilate: a new oral thrombin inhibitor. Circulation. 2011; 123:1436–1450. PMID: 21464059.6. Kakar P, Watson T, Lip GY. Drug evaluation: rivaroxaban, an oral, direct inhibitor of activated factor X. Curr Opin Investig Drugs. 2007; 8:256–265.7. Ross JS, Stagliano NE, Donovan MJ, Breitbart RE, Ginsburg GS. Atherosclerosis and cancer: common molecular pathways of disease development and progression. Ann N Y Acad Sci. 2001; 947:271–292. PMID: 11795276.8. Forood A, Malekpour-Afshar R, Mahdavi A. Serum level of plasminogen activator inhibitor type-1 in addicted patients with coronary artery disease. Addict Health. 2014; 6:119–126. PMID: 25984279.9. Wang TJ, Gona P, Larson MG, et al. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med. 2006; 355:2631–2639. PMID: 17182988.
Article10. Stangier J, Clemens A. Pharmacology, pharmacokinetics, and pharmacodynamics of dabigatran etexilate, an oral direct thrombin inhibitor. Clin Appl Thromb Hemost. 2009; 15 Suppl 1:9S–16S. PMID: 19696042.
Article11. Stangier J, Rathgen K, Stähle H, Gansser D, Roth W. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol. 2007; 64:292–303. PMID: 17506785.
Article12. Ozmen S, Ayhan S, Demir Y, Siemionow M, Atabay K. Impact of gradual blood flow increase on ischaemia-reperfusion injury in the rat cremaster microcirculation model. J Plast Reconstr Aesthet Surg. 2008; 61:939–948. PMID: 17632046.
Article13. Yamashita T, Shoge M, Oda E, et al. The free-radical scavenger, edaravone, augments NO release from vascular cells and platelets after laser-induced, acute endothelial injury in vivo. Platelets. 2006; 17:201–206. PMID: 16702048.14. Yamashita T, Oda E, Sano T, et al. Varying the ratio of dietary n-6/n-3 polyunsaturated fatty acid alters the tendency to thrombosis and progress of atherosclerosis in apoE−/− LDLR−/− double knockout mouse. Thromb Res. 2005; 116:393–401. PMID: 16122552.15. Jennings LK. Mechanisms of platelet activation: need for new strategies to protect against platelet-mediated atherothrombosis. Thromb Haemost. 2009; 102:248–257. PMID: 19652875.
Article16. Larsen TB, Skjøth F, Nielsen PB, Kjældgaard JN, Lip GY. Comparative effectiveness and safety of non-vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. BMJ. 2016; 353:i3189. PMID: 27312796.
Article17. Wienen W, Stassen JM, Priepke H, Ries UJ, Hauel N. Effects of the direct thrombin inhibitor dabigatran and its orally active prodrug, dabigatran etexilate, on thrombus formation and bleeding time in rats. Thromb Haemost. 2007; 98:333–338. PMID: 17721615.
Article18. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009; 361:1139–1151. PMID: 19717844.
Article19. Fusaro M, Dalle Carbonare L, Dusso A, et al. Differential effects of dabigatran and warfarin on bone volume and structure in rats with normal renal function. PLoS One. 2015; 10:e0133847. PMID: 26241483.
Article20. Bouchard L, Vohl MC, Lebel S, et al. Contribution of genetic and metabolic syndrome to omental adipose tissue PAI-1 gene mRNA and plasma levels in obesity. Obes Surg. 2010; 20:492–499. PMID: 20127289.
Article21. Du HQ, Yin M, Ye HY, et al. Expression profiles of lipid metabolism-related genes in liver of apoE−/−/LDLR−/− mice. Zhonghua Bing Li Xue Za Zhi. 2007; 36:751–755. PMID: 18307880.22. Tsantes AE, Nikolopoulos GK, Bagos PG, Bonovas S, Kopterides P, Vaiopoulos G. The effect of the plasminogen activator inhibitor-1 4G/5G polymorphism on the thrombotic risk. Thromb Res. 2008; 122:736–742. PMID: 17949795.
Article23. Zhu Y, Carmeliet P, Fay WP. Plasminogen activator inhibitor-1 is a major determinant of arterial thrombolysis resistance. Circulation. 1999; 99:3050–3055. PMID: 10368124.
Article24. Sinkovic A, Pogacar V. Risk stratification in patients with unstable angina and/or non-ST-elevation myocardial infarction by troponin T and plasminogen-activator-inhibitor-1 (PAI-1). Thromb Res. 2004; 114:251–257. PMID: 15381388.
Article25. Zunker P, Schick A, Padró T, Kienast J, Phillips A, Ringelstein EB. Tissue plasminogen activator and plasminogen activator inhibitor in patients with acute ischemic stroke: relation to stroke etiology. Neurol Res. 1999; 21:727–732. PMID: 10596380.
Article26. Boulaftali Y, Ho-Tin-Noe B, Pena A, et al. Platelet protease nexin-1, a serpin that strongly influences fibrinolysis and thrombolysis. Circulation. 2011; 123:1326–1334. PMID: 21403095.
Article27. Danckwardt S, Hentze MW, Kulozik AE. Pathologies at the nexus of blood coagulation and inflammation: thrombin in hemostasis, cancer, and beyond. J Mol Med (Berl). 2013; 91:1257–1271. PMID: 23955016.
Article28. Vercauteren E, Peeters M, Hoylaerts MF, et al. The hyperfibrinolytic state of mice with combined thrombin-activatable fibrinolysis inhibitor (TAFI) and plasminogen activator inhibitor-1 gene deficiency is critically dependent on TAFI deficiency. J Thromb Haemost. 2012; 10:2555–2562. PMID: 23083123.
Article29. Moncada S, Higgs EA. Nitric oxide and the vascular endothelium. Handb Exp Pharmacol. 2006; 176:213–254.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hemopericardium and Cardiac Tamponade in a Patient Treated with Dabigatran Etexilate
- Subject The Effect of Apolipoprotein E Overexpression on Plasma Lipoprotein Profile in Mice Fed on Long-term High Cholesterol Diet
- Inhibitory effects of tilianin on the expression of inducible nitric oxide synthase in low density lipoprotein receptor deficiency mice
- Effects of FXR Deficiency and Pioglitazone on Atherosclerosis in ApoE-Knockout Mice
- The enhanced expression of IL-17-secreting T cells during the early progression of atherosclerosis in ApoE-deficient mice fed on a western-type diet