Clin Endosc.
2020 Jul;53(4):417-428. 10.5946/ce.2019.053.
Endoscopic Ultrasound-Guided Fine Needle Aspiration and Endoscopic Retrograde Cholangiopancreatography-Based Tissue Sampling in Suspected Malignant Biliary Strictures: A Meta-Analysis of Same-Session Procedures
- Affiliations
-
- 1Division of Gastroenterology, Hepatology and Endoscopy, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA
- 2Endoscopy Unit, Department of Gastroenterology, Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo, Brazil
- KMID: 2504945
- DOI: http://doi.org/10.5946/ce.2019.053
Abstract
- Background/Aims
The diagnosis of biliary strictures can be challenging. There are no systematic reviews studying same-session endoscopic retrograde cholangiopancreatography (ERCP)-based tissue sampling and endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) for the diagnosis of biliary strictures.
Methods
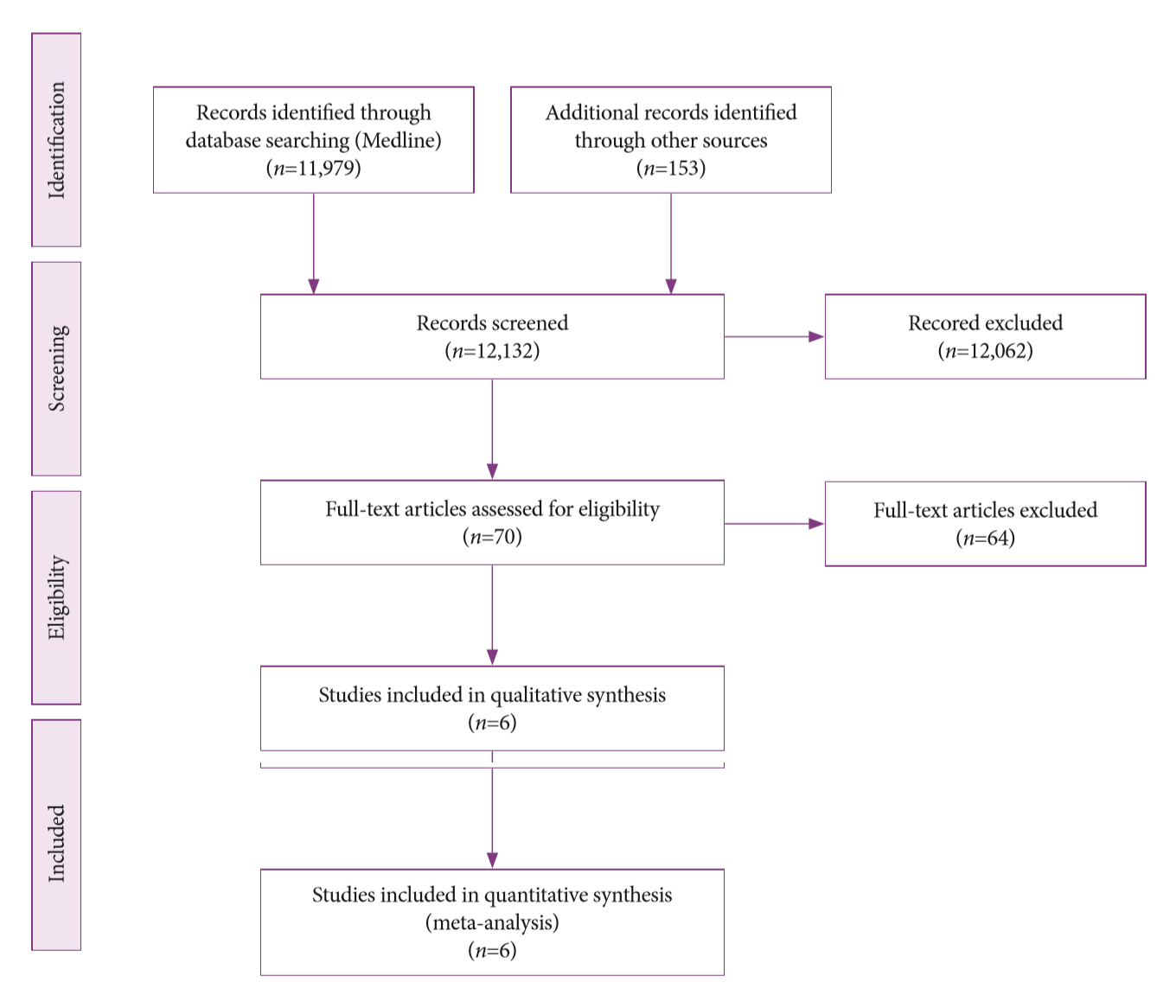
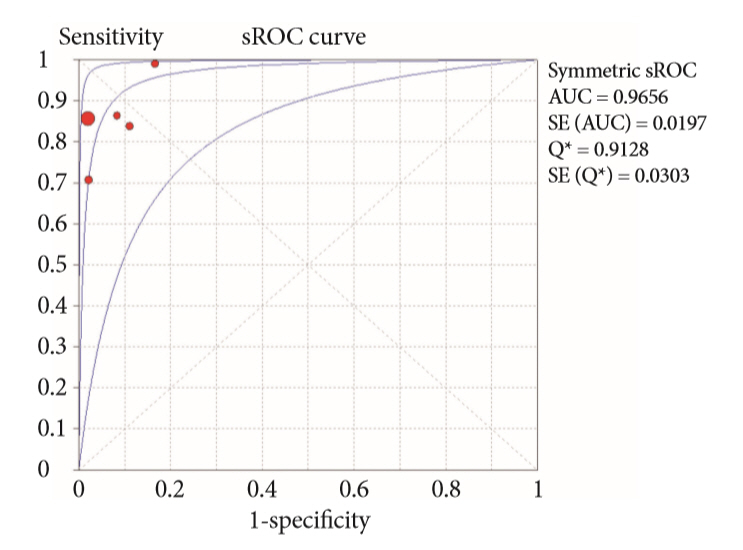
A systematic review was conducted on studies analyzing same-session EUS and ERCP for tissue diagnosis of suspected malignant biliary strictures. The primary outcome was the accuracy of each method individually compared to the two methods combined. The secondary outcome was the accuracy of each method in pancreatic and biliary etiologies. In the meta-analysis, we used Forest plots, summary receiver operating characteristic curves, and estimates of the area under the curve for intention-to-treat analysis.
Results
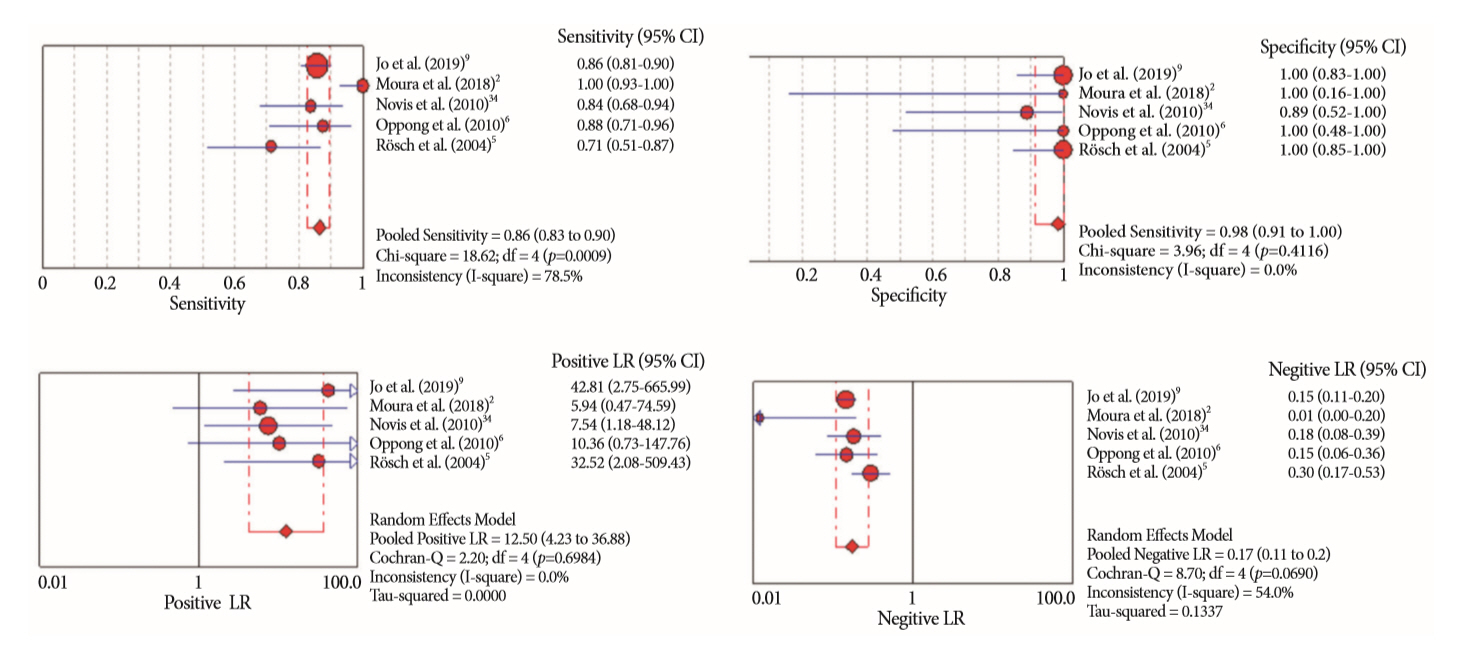
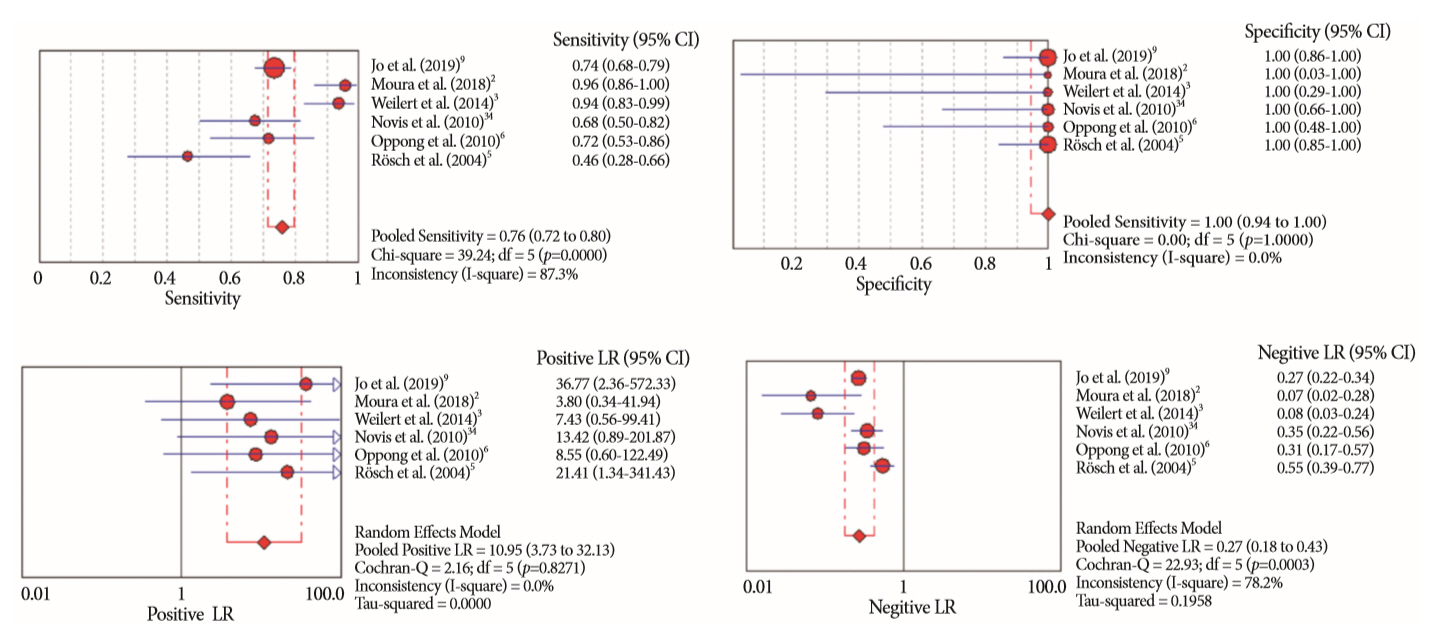
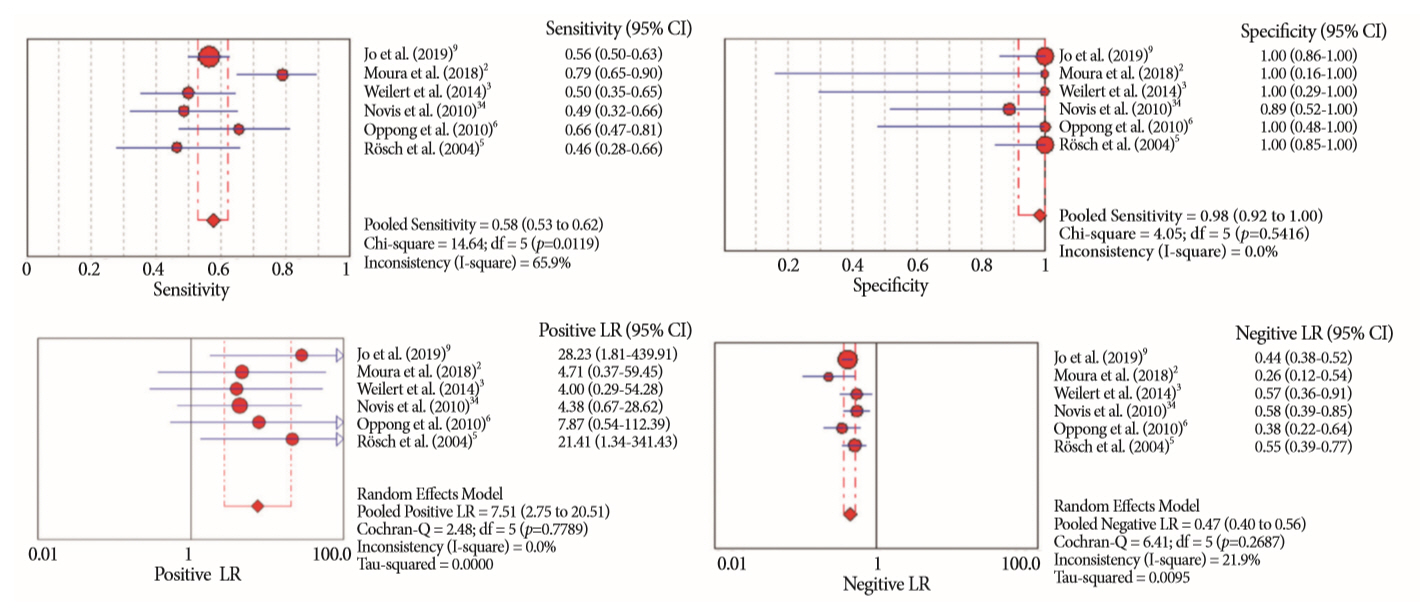
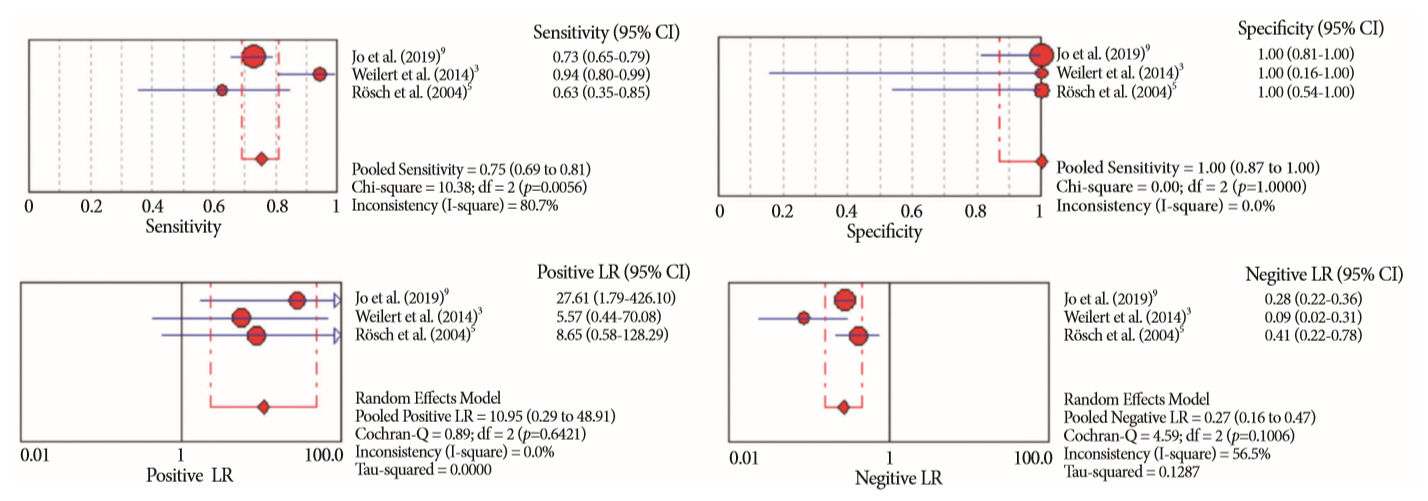
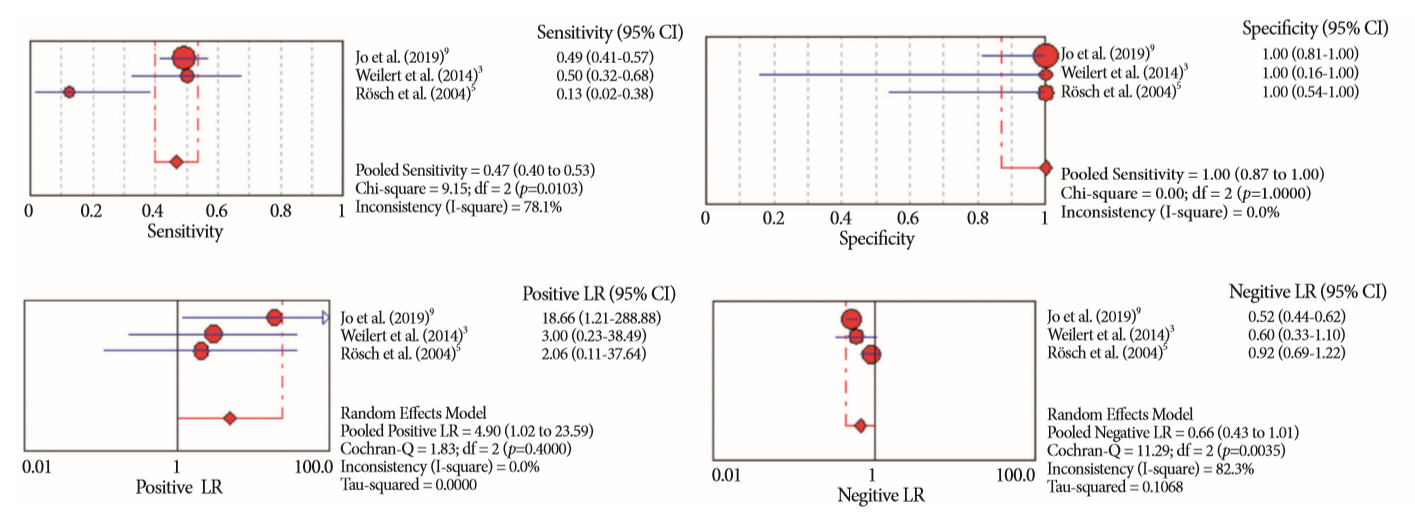
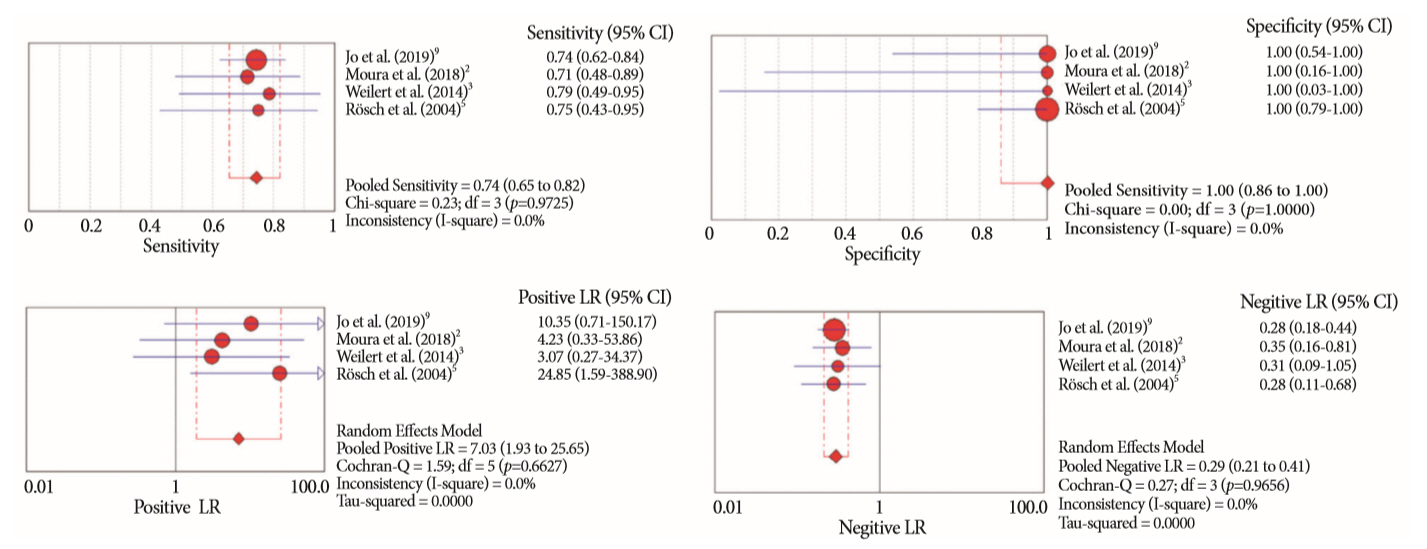
Of the 12,132 articles identified, six were included, resulting in a total of 497 patients analyzed. The sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, and accuracy of the association between the two methods were: 86%, 98%, 12.50, 0.17, and 96.5%, respectively. For the individual analysis, the sensitivity, specificity and accuracy of EUS-FNA were 76%, 100%, and 94.5%, respectively; for ERCP-based tissue sampling, the sensitivity, specificity, and accuracy were 58%, 98%, and 78.1%, respectively. For pancreatic lesions, EUS-FNA was superior to ERCP-based tissue sampling. However, for biliary lesions, both methods had similar sensitivities.
Conclusions
Same-session EUS-FNA and ERCP-based tissue sampling is superior to either method alone in the diagnosis of suspected malignant biliary strictures. Considering these results, combination sampling should be performed when possible.
Keyword
Figure
Cited by 1 articles
-
Stent versus Balloon Dilation for the Treatment of Dominant Strictures in Primary Sclerosing Cholangitis: A Systematic Review and Meta-Analysis
Marina Tucci Gammaro Baldavira Ferreira, Igor Braga Ribeiro, Diogo Turiani Hourneaux de Moura, Thomas R. McCarty, Alberto Machado da Ponte Neto, Galileu Ferreira Ayala Farias, Antônio Afonso de Miranda Neto, Pedro Victor Aniz Gomes de Oliveira, Wanderley Marques Bernardo, Eduardo Guimarães Hourneaux de Moura
Clin Endosc. 2021;54(6):833-842. doi: 10.5946/ce.2021.052.
Reference
-
1. Korc P, Sherman S. ERCP tissue sampling. Gastrointest Endosc. 2016; 84:557–571.
Article2. Moura DTH, de Moura EGH, Matuguma SE, et al. EUS-FNA versus ERCP for tissue diagnosis of suspect malignant biliary strictures: a prospective comparative study. Endosc Int Open. 2018; 6:E769–E777.
Article3. Weilert F, Bhat YM, Binmoeller KF, et al. EUS-FNA is superior to ERCP-based tissue sampling in suspected malignant biliary obstruction: results of a prospective, single-blind, comparative study. Gastrointest Endosc. 2014; 80:97–104.
Article4. American Society for Gastrointestinal Endoscopy (ASGE) Standards of Practice Committee, Anderson MA, Appalaneni V, et al. The role of endoscopy in the evaluation and treatment of patients with biliary neoplasia. Gastrointest Endosc. 2013; 77:167–174.
Article5. Rösch T, Hofrichter K, Frimberger E, et al. ERCP or EUS for tissue diagnosis of biliary strictures? A prospective comparative study. Gastrointest Endosc. 2004; 60:390–396.
Article6. Oppong K, Raine D, Nayar M, Wadehra V, Ramakrishnan S, Charnley RM. EUS-FNA versus biliary brushings and assessment of simultaneous performance in jaundiced patients with suspected malignant obstruction. JOP. 2010; 11:560–567.7. Butturini G, Stocken DD, Wente MN, et al. Influence of resection margins and treatment on survival in patients with pancreatic cancer: meta-analysis of randomized controlled trials. Arch Surg. 2008; 143:75–83. discussion 83.8. Mansfield SD, Barakat O, Charnley RM, et al. Management of hilar cholangiocarcinoma in the North of England: pathology, treatment, and outcome. World J Gastroenterol. 2005; 11:7625–7630.
Article9. Jo JH, Cho CM, Jun JH, et al. Same-session endoscopic ultrasound-guided fine needle aspiration and endoscopic retrograde cholangiopancreatography-based tissue sampling in suspected malignant biliary obstruction: a multicenter experience. J Gastroenterol Hepatol. 2019; 34:799–805.
Article10. Jailwala J, Fogel EL, Sherman S, et al. Triple-tissue sampling at ERCP in malignant biliary obstruction. Gastrointest Endosc. 2000; 51(4 Pt 1):383–390.
Article11. Shieh FK, Luong-Player A, Khara HS, et al. Improved endoscopic retrograde cholangiopancreatography brush increases diagnostic yield of malignant biliary strictures. World J Gastrointest Endosc. 2014; 6:312–317.
Article12. Ponchon T, Gagnon P, Berger F, et al. Value of endobiliary brush cytology and biopsies for the diagnosis of malignant bile duct stenosis: results of a prospective study. Gastrointest Endosc. 1995; 42:565–572.
Article13. Pugliese V, Conio M, Nicolò G, Saccomanno S, Gatteschi B. Endoscopic retrograde forceps biopsy and brush cytology of biliary strictures: a prospective study. Gastrointest Endosc. 1995; 42:520–526.
Article14. Draganov PV, Chauhan S, Wagh MS, et al. Diagnostic accuracy of conventional and cholangioscopy-guided sampling of indeterminate biliary lesions at the time of ERCP: a prospective, long-term follow-up study. Gastrointest Endosc. 2012; 75:347–353.
Article15. Glasbrenner B, Ardan M, Boeck W, Preclik G, Möller P, Adler G. Prospective evaluation of brush cytology of biliary strictures during endoscopic retrograde cholangiopancreatography. Endoscopy. 1999; 31:712–717.
Article16. Sugiyama M, Atomi Y, Wada N, Kuroda A, Muto T. Endoscopic transpapillary bile duct biopsy without sphincterotomy for diagnosing biliary strictures: a prospective comparative study with bile and brush cytology. Am J Gastroenterol. 1996; 91:465–467.17. Farrell RJ, Jain AK, Brandwein SL, Wang H, Chuttani R, Pleskow DK. The combination of stricture dilation, endoscopic needle aspiration, and biliary brushings significantly improves diagnostic yield from malignant bile duct strictures. Gastrointest Endosc. 2001; 54:587–594.
Article18. Wiersema M, Lehman G, Hawes R. Improvement of diagnostic yield of brush cytology in malignant strictures by the use of supplemental tissue sampling technique. Gastrointest Endosc. 1992; 35:265A.19. Singh H, Siddiqui AA. Endosonographic workup and preoperative biliary drainage for pancreatic cancer. Semin Oncol. 2015; 42:59–69.
Article20. Harinck F, Konings IC, Kluijt I, et al. A multicentre comparative prospective blinded analysis of EUS and MRI for screening of pancreatic cancer in high-risk individuals. Gut. 2016; 65:1505–1513.
Article21. Dewitt J, Devereaux BM, Lehman GA, Sherman S, Imperiale TF. Comparison of endoscopic ultrasound and computed tomography for the preoperative evaluation of pancreatic cancer: a systematic review. Clin Gastroenterol Hepatol. 2006; 4:717–725. quiz 664.
Article22. Guedes HG, Moura DTH, Duarte RB, et al. A comparison of the efficiency of 22G versus 25G needles in EUS-FNA for solid pancreatic mass assessment: a systematic review and meta-analysis. Clinics (Sao Paulo). 2018; 73:e261.
Article23. De Moura DT, Chacon DA, Tanigawa R, et al. Pancreatic metastases from ocular malignant melanoma: the use of endoscopic ultrasound-guided fine-needle aspiration to establish a definitive cytologic diagnosis: a case report. J Med Case Rep. 2016; 10:332.
Article24. De Moura DTH, Moura EGH, Bernardo WM, et al. Endoscopic retrograde cholangiopancreatography versus endoscopic ultrasound for tissue diagnosis of malignant biliary stricture: systematic review and meta-analysis. Endosc Ultrasound. 2018; 7:10–19.
Article25. Wang W, Shpaner A, Krishna SG, et al. Use of EUS-FNA in diagnosing pancreatic neoplasm without a definitive mass on CT. Gastrointest Endosc. 2013; 78:73–80.
Article26. Mohamadnejad M, DeWitt JM, Sherman S, et al. Role of EUS for preoperative evaluation of cholangiocarcinoma: a large single-center experience. Gastrointest Endosc. 2011; 73:71–78.
Article27. Fritscher-Ravens A, Broering DC, Knoefel WT, et al. EUS-guided fine-needle aspiration of suspected hilar cholangiocarcinoma in potentially operable patients with negative brush cytology. Am J Gastroenterol. 2004; 99:45–51.
Article28. Eloubeidi MA, Chen VK, Jhala NC, et al. Endoscopic ultrasound-guided fine needle aspiration biopsy of suspected cholangiocarcinoma. Clin Gastroenterol Hepatol. 2004; 2:209–213.
Article29. DeWitt J, Misra VL, Leblanc JK, McHenry L, Sherman S. EUS-guided FNA of proximal biliary strictures after negative ERCP brush cytology results. Gastrointest Endosc. 2006; 64:325–333.
Article30. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009; 151:W65–W94.
Article31. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009; 6:e1000097.
Article32. Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011; 155:529–536.
Article33. Centre for Evidence-Based Medicine. CATMaker and EBM calculators [Internet]. Oxford;CEBM: c2018 [cited 2018 Dec 7]. Available from: https://www.cebm.net/2014/06/catmaker-ebm-calculators/.34. Novis M, Ardengh JC, Libera ED, et al. [Prospective comparative study of ERCP brush cytology and EUS-FNA for the differential diagnosis of biliary strictures]. Rev Col Bras Cir. 2010; 37:190–198.35. Prat F. Suspected malignant biliary strictures: from inside out or outside in? Endosc Int Open. 2018; 6:E778–E779.
Article36. Navaneethan U, Njei B, Lourdusamy V, Konjeti R, Vargo JJ, Parsi MA. Comparative effectiveness of biliary brush cytology and intraductal biopsy for detection of malignant biliary strictures: a systematic review and meta-analysis. Gastrointest Endosc. 2015; 81:168–176.
Article37. Naitoh I, Nakazawa T, Kato A, et al. Predictive factors for positive diagnosis of malignant biliary strictures by transpapillary brush cytology and forceps biopsy. J Dig Dis. 2016; 17:44–51.
Article38. De Moura DTH, Coronel M, Chacon DA, et al. Primary adenosquamous cell carcinoma of the pancreas: the use of endoscopic ultrasound guided - fine needle aspiration to establish a definitive cytologic diagnosis. Rev Gastroenterol Peru. 2017; 37:370–373.39. De Moura DTH, Coronel M, Ribeiro IB, et al. The importance of endoscopic ultrasound fine-needle aspiration in the diagnosis of solid pseudopapillary tumor of the pancreas: two case reports. J Med Case Rep. 2018; 12:107.
Article40. Banales JM, Cardinale V, Carpino G, et al. Expert consensus document: cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol. 2016; 13:261–280.41. Matynia AP, Schmidt RL, Barraza G, Layfield LJ, Siddiqui AA, Adler DG. Impact of rapid on-site evaluation on the adequacy of endoscopic-ultrasound guided fine-needle aspiration of solid pancreatic lesions: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2014; 29:697–705.
Article42. Bang JY, Kirtane S, Krall K, et al. In memoriam: fine-needle aspiration, birth: fine-needle biopsy: the changing trend in endoscopic ultrasound-guided tissue acquisition. Dig Endosc. 2019; 31:197–202.
Article43. Brooks C, Gausman V, Kokoy-Mondragon C, et al. Role of fluorescent in situ hybridization, cholangioscopic biopsies, and EUS-FNA in the evaluation of biliary strictures. Dig Dis Sci. 2018; 63:636–644.
Article44. Navaneethan U, Hasan MK, Lourdusamy V, Njei B, Varadarajulu S, Hawes RH. Single-operator cholangioscopy and targeted biopsies in the diagnosis of indeterminate biliary strictures: a systematic review. Gastrointest Endosc. 2015; 82:608–614.e2.
Article45. Heimbach JK, Sanchez W, Rosen CB, Gores GJ. Trans-peritoneal fine needle aspiration biopsy of hilar cholangiocarcinoma is associated with disease dissemination. HPB (Oxford). 2011; 13:356–360.
Article46. El Chafic AH, Dewitt J, Leblanc JK, et al. Impact of preoperative endoscopic ultrasound-guided fine needle aspiration on postoperative recurrence and survival in cholangiocarcinoma patients. Endoscopy. 2013; 45:883–889.
Article47. Onda S, Ogura T, Kurisu Y, et al. EUS-guided FNA for biliary disease as first-line modality to obtain histological evidence. Therap Adv Gastroenterol. 2016; 9:302–312.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of Repeated Endoscopic Ultrasound-Guided Fine Needle Aspiration for Inconclusive Initial Cytology Result
- Comparison of the Diagnostic Performances of Same-session Endoscopic Ultrasound- and Endoscopic Retrograde Cholangiopancreatography-guided Tissue Sampling for Suspected Biliary Strictures at Different Primary Tumor Sites
- Advances of Peroral Cholangioscopy and EUS for Indeterminate Biliary Lesions
- Endoscopic methods for cytopathologic diagnosis of bile duct strictures
- Endoscopic Ultrasonography in the Evaluation of Indeterminate Biliary Strictures