J Pathol Transl Med.
2020 Jul;54(4):290-299. 10.4132/jptm.2020.05.04.
Peripheral type squamous cell carcinoma of the lung: clinicopathologic characteristics in comparison to the central type
- Affiliations
-
- 1Department of Hospital Pathology, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 2Department of Hospital Pathology, St. Vincent’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2504559
- DOI: http://doi.org/10.4132/jptm.2020.05.04
Abstract
- Background
Squamous cell carcinomas (SqCCs) of the lung are known to arise more often in a central area but reports of peripheral SqCCs have increased, with a pathogenesis that is obscured. In this study, the clinicopathologic characteristics of peripheral lung SqCCs were studied and compared with those of the central type.
Methods
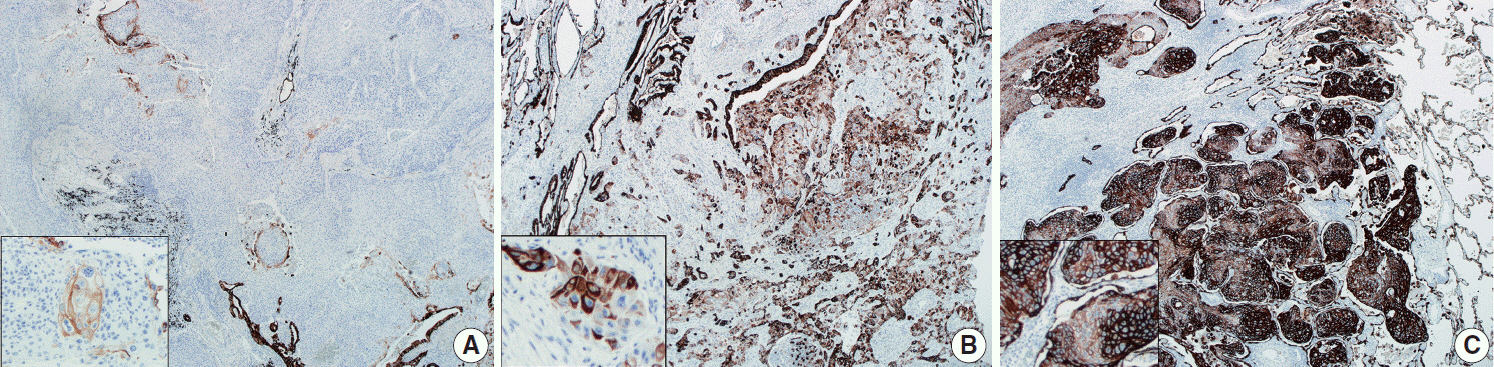
This study included 63 peripheral lung SqCCs and 48 randomly selected central cases; hematoxylin and eosin-stained slides of surgically resected specimens were reviewed in conjunction with radiologic images and clinical history. Cytokeratin-7 immunohistochemical staining of key slides and epidermal growth factor receptor (EGFR)/KRAS mutations tested by DNA sequencing were also included.
Results
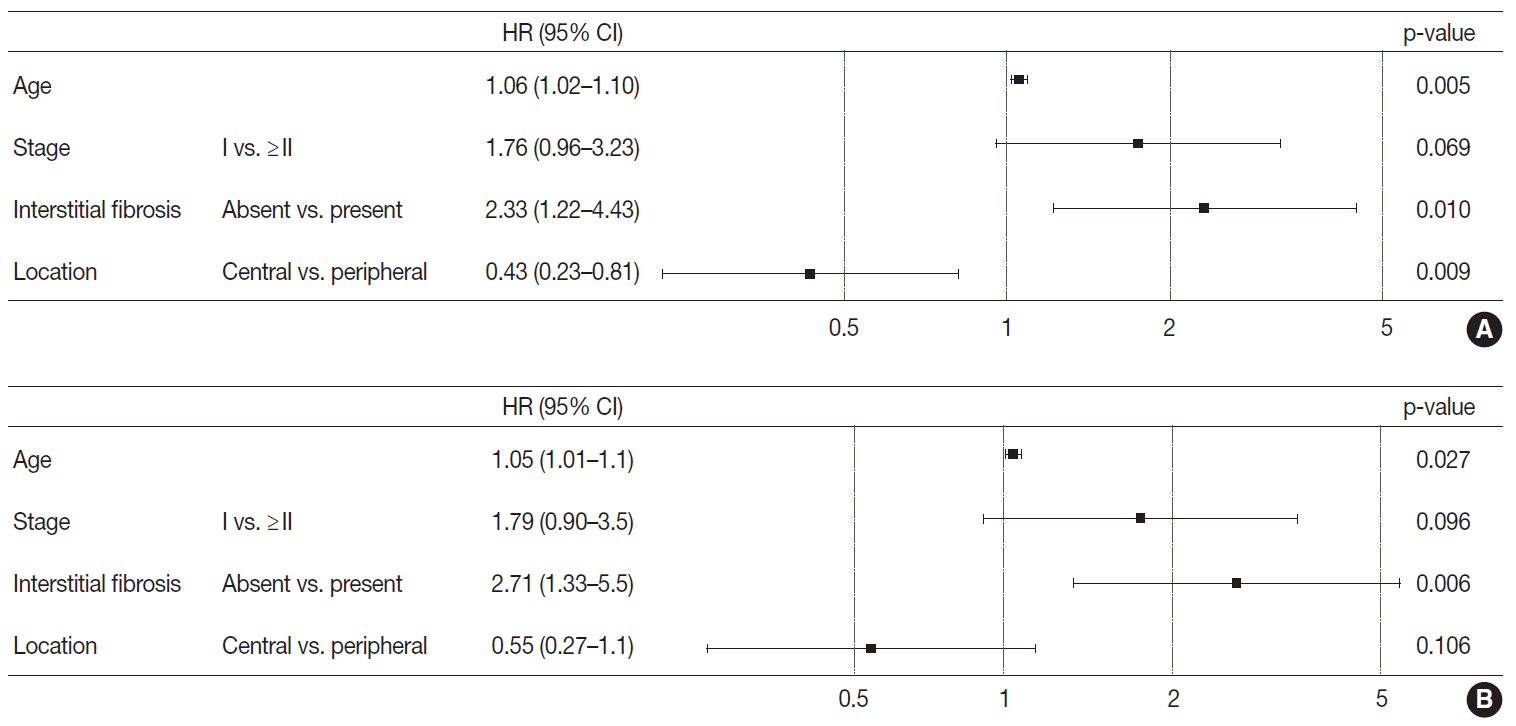
Stages of peripheral SqCCs were significantly lower than central SqCCs (p=.016). Cystic change of the mass (p=.007), presence of interstitial fibrosis (p=0.007), and anthracosis (p=.049) in the background lung were significantly associated with the peripheral type. Cytokeratin-7 positivity was also higher in peripheral SqCCs with cutoffs of both 10% and 50% (p=.011). Pathogenic mutations in EGFR and KRAS were observed in only one case out of the 72 evaluated. The Cox proportional hazard model indicated a significantly better disease-free survival (p=.009) and the tendency of better overall survival (p=.106) in the peripheral type.
Conclusions
In peripheral type, lower stage is a favorable factor for survival but more frequent interstitial fibrosis and older age are unfavorable factors. Multivariate Cox analysis revealed that peripheral type is associated with better disease-free survival. The pathogenesis of peripheral lung SqCCs needs further investigation, together with consideration of the background lung conditions.
Figure
Reference
-
References
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015; 65:87–108.
Article2. Colby TV, Koss KM, Travis WD. Tumors of the lower respiratory tract, fascicle 13. Washington, DC: Armed Forces Institute of Pathology;1995.3. Funai K, Yokose T, Ishii G, et al. Clinicopathologic characteristics of peripheral squamous cell carcinoma of the lung. Am J Surg Pathol. 2003; 27:978–84.
Article4. Brooks DR, Austin JH, Heelan RT, et al. Influence of type of cigarette on peripheral versus central lung cancer. Cancer Epidemiol Biomarkers Prev. 2005; 14:576–81.
Article5. Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. WHO classification of tumours of the lung, pleura, thymus and heart. Lyon: International Agency for Research on Cancer (IARC);2015.6. Yamano S, Gi M, Tago Y, et al. Role of deltaNp63(pos)CD44v(pos) cells in the development of N-nitroso-tris-chloroethylurea-induced peripheral-type mouse lung squamous cell carcinomas. Cancer Sci. 2016; 107:123–32.7. Dai YB, Miao YF, Wu WF, et al. Ablation of Liver X receptors alpha and beta leads to spontaneous peripheral squamous cell lung cancer in mice. Proc Natl Acad Sci U S A. 2016; 113:7614–9.8. Sakurai H, Asamura H, Watanabe S, Suzuki K, Tsuchiya R. Clinicopathologic features of peripheral squamous cell carcinoma of the lung. Ann Thorac Surg. 2004; 78:222–7.
Article9. Hayashi T, Sano H, Egashira R, et al. Difference of morphology and immunophenotype between central and peripheral squamous cell carcinomas of the lung. Biomed Res Int. 2013; 2013:157838.
Article10. Saijo T, Ishii G, Nagai K, et al. Differences in clinicopathological and biological features between central-type and peripheral-type squamous cell carcinoma of the lung. Lung Cancer. 2006; 52:37–45.
Article11. Shimosato Y, Suzuki A, Hashimoto T, et al. Prognostic implications of fibrotic focus (scar) in small peripheral lung cancers. Am J Surg Pathol. 1980; 4:365–73.
Article12. R Core Team. R: a language and environment for statistical computing [Internet]. Vienna: R Foundation for Statistical Computing;2018. [cited 2019 Dec 24]. Available from: https://www.R-project.org/.13. Sheard S, Moser J, Sayer C, Stefanidis K, Devaraj A, Vlahos I. Lung cancers associated with cystic airspaces: underrecognized features of early disease. Radiographics. 2018; 38:704–17.14. Mascalchi M, Attina D, Bertelli E, et al. Lung cancer associated with cystic airspaces. J Comput Assist Tomogr. 2015; 39:102–8.
Article15. Yousem SA. Peripheral squamous cell carcinoma of lung: patterns of growth with particular focus on airspace filling. Hum Pathol. 2009; 40:861–7.
Article16. van de Molengraft FJ, van Niekerk CC, Jap PH, Poels LG. OV-TL 12/30 (keratin 7 antibody) is a marker of glandular differentiation in lung cancer. Histopathology. 1993; 22:35–8.
Article17. Baars JH, De Ruijter JL, Smedts F, et al. The applicability of a keratin 7 monoclonal antibody in routinely Papanicolaou-stained cytologic specimens for the differential diagnosis of carcinomas. Am J Clin Pathol. 1994; 101:257–61.
Article18. van Niekerk CC, Jap PH, Ramaekers FC, van de Molengraft F, Poels LG. Immunohistochemical demonstration of keratin 7 in routinely fixed paraffin-embedded human tissues. J Pathol. 1991; 165:145–52.
Article19. Johansson L. Histopathologic classification of lung cancer: relevance of cytokeratin and TTF-1 immunophenotyping. Ann Diagn Pathol. 2004; 8:259–67.
Article20. Xu Y, Wang W, Li L, et al. FOXA1 and CK7 expression in esophageal squamous cell carcinoma and its prognostic significance. Neoplasma. 2018; 65:469–76.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Characteristics of Peripheral versus Central Lung Cancer Since 2000
- Immunohistochemical Assessment of Peripheral Squamous Lung Cancer: Comparison to Central Squamous Cell Carcinoma and Peripheral Adenocarcinoma
- The Role of CT in the Diagnosis of Bronchogenic Carcinoma not Detected by Plain Radiograph
- Basaloid Squamous Cell Carcinoma of the Lung: Two Case Reports with CT Imaging Findings
- The National Survey of Lung Cancer in Korea