Cancer Res Treat.
2020 Jul;52(3):830-847. 10.4143/crt.2019.510.
Activation of β2-Adrenergic Receptor Promotes Growth and Angiogenesis in Breast Cancer by Down-regulating PPARγ
- Affiliations
-
- 1Department of Immunology, School of Medicine, Nankai University, Tianjin, China
- 2Department of Pediatrics, Tianjin Nankai Hospital, Tianjin, China
- 3Tianjin Key Laboratory of Oral and Maxillofacial Function Reconstruction, Hospital of Stomatology, Nankai University, Tianjin, China
- 4State Key Laboratory of Medicinal Chemical Biology, Nankai University, Tianjin, China
- KMID: 2504463
- DOI: http://doi.org/10.4143/crt.2019.510
Abstract
- Purpose
Chronic stress and related hormones are key in cancer progression. Peroxisome proliferator-activated receptor γ (PPARγ) and its agonists was reported that inducing anti-tumor effect. However, the function of PPARγ in pro-tumorigenic effects induced by chronic stress in breast cancer remains unknown. Herein, we have characterized a novel role of PPARγ and vascular endothelial growth factor (VEGF)/fibroblast growth factor 2 (FGF2) signals in breast cancer promoted by chronic stress.
Materials and Methods
We performed experiments in vivo and in vitro and used bioinformatics data to evaluate the therapeutic potential of PPARγ in breast cancer promoted by stress.
Results
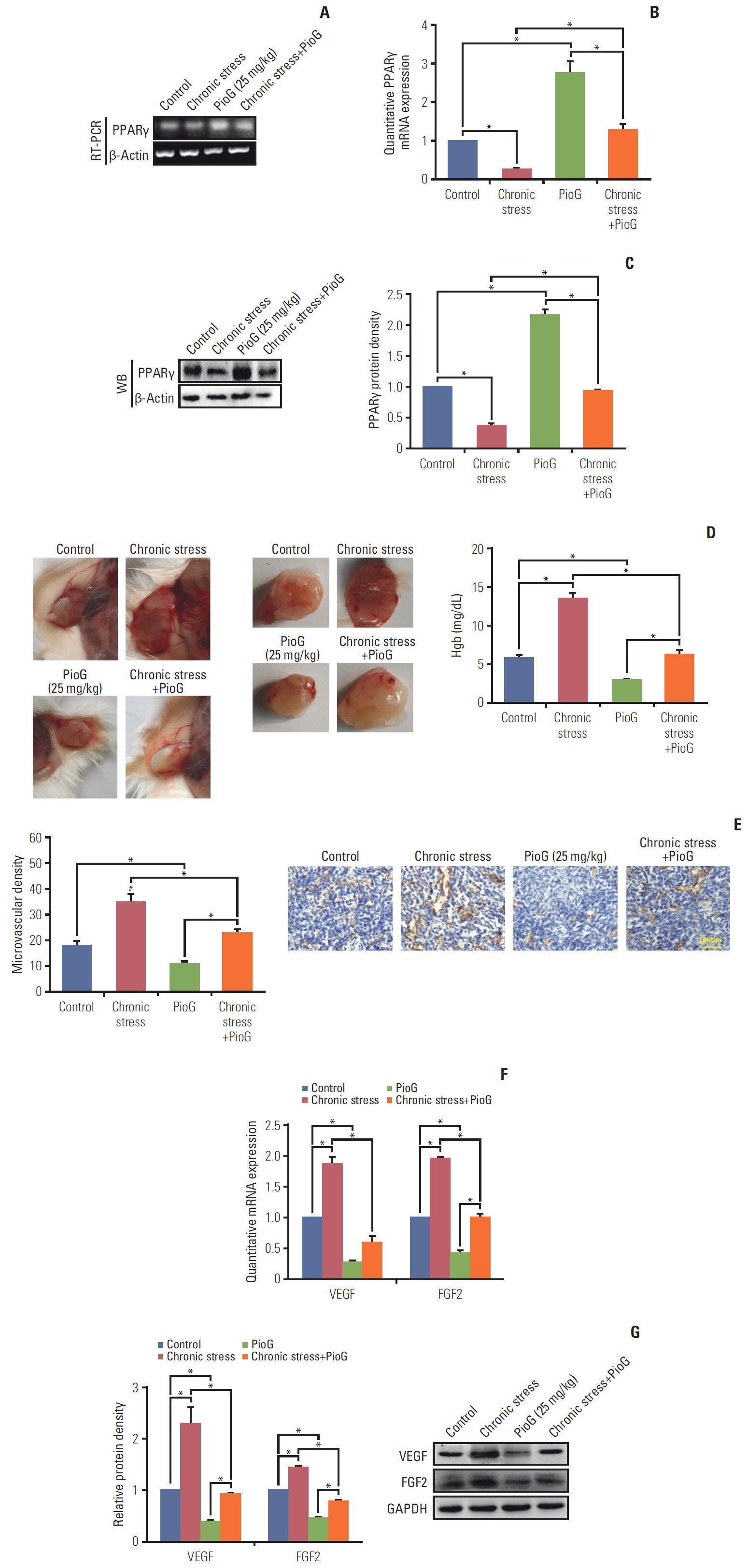
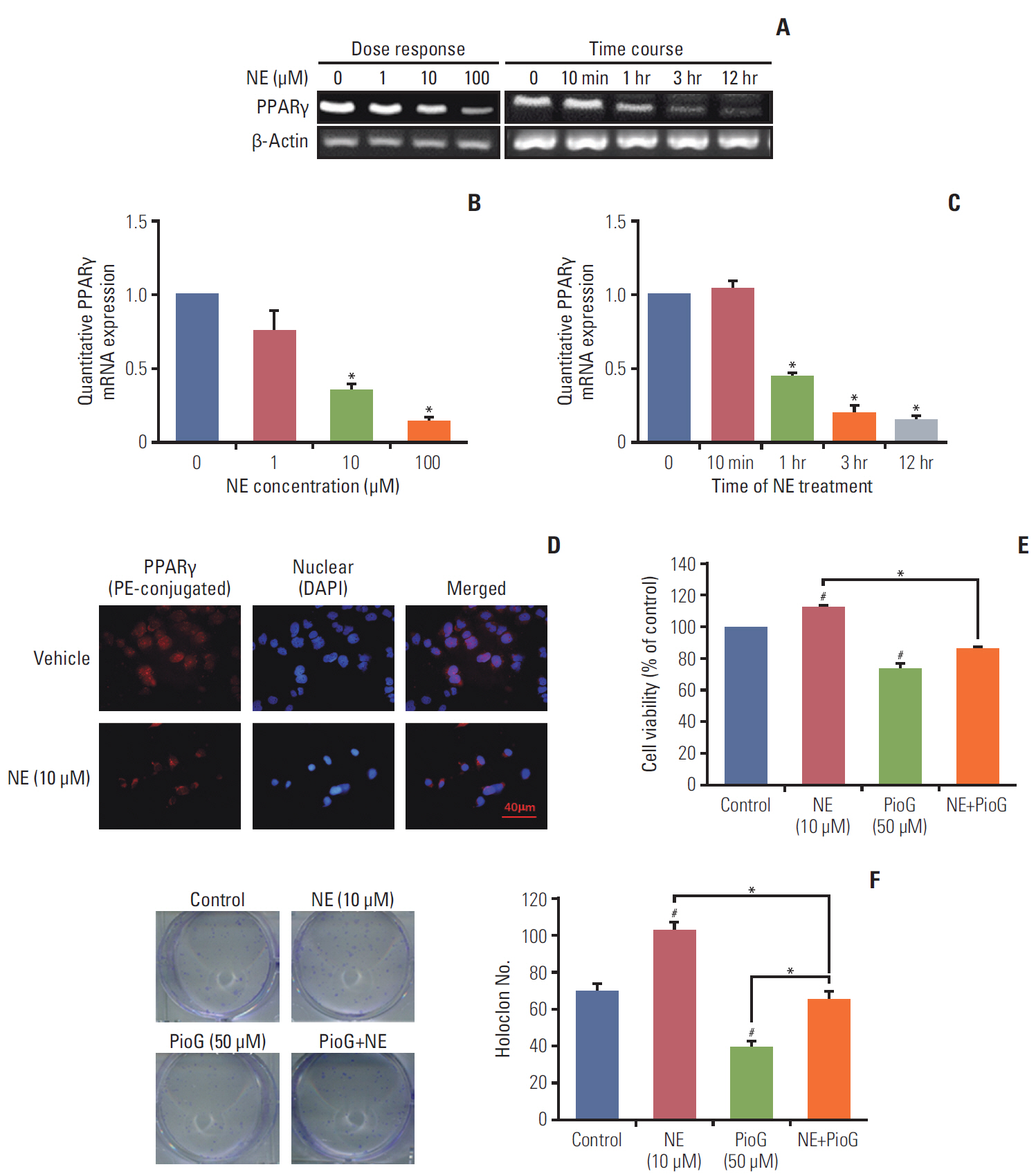
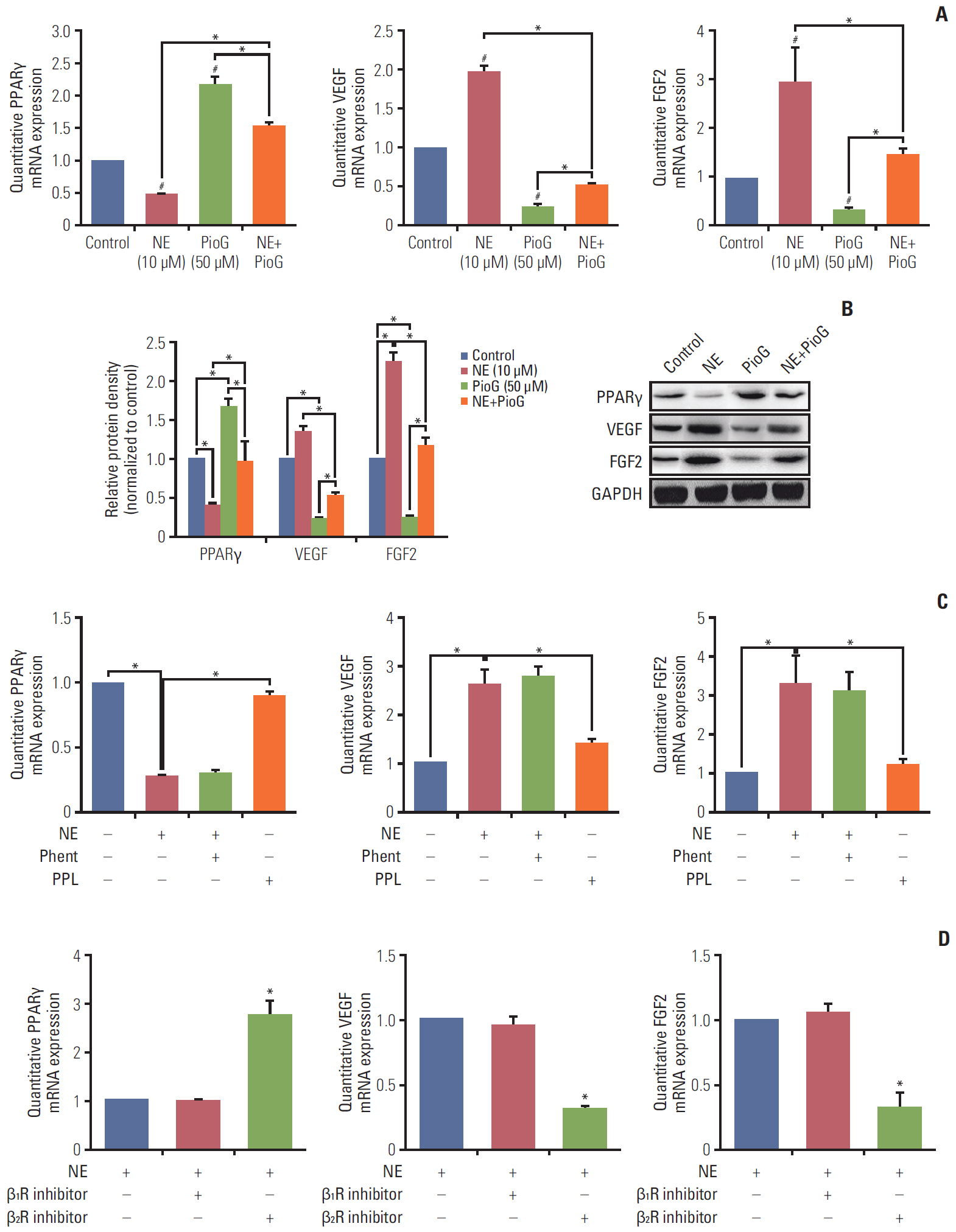
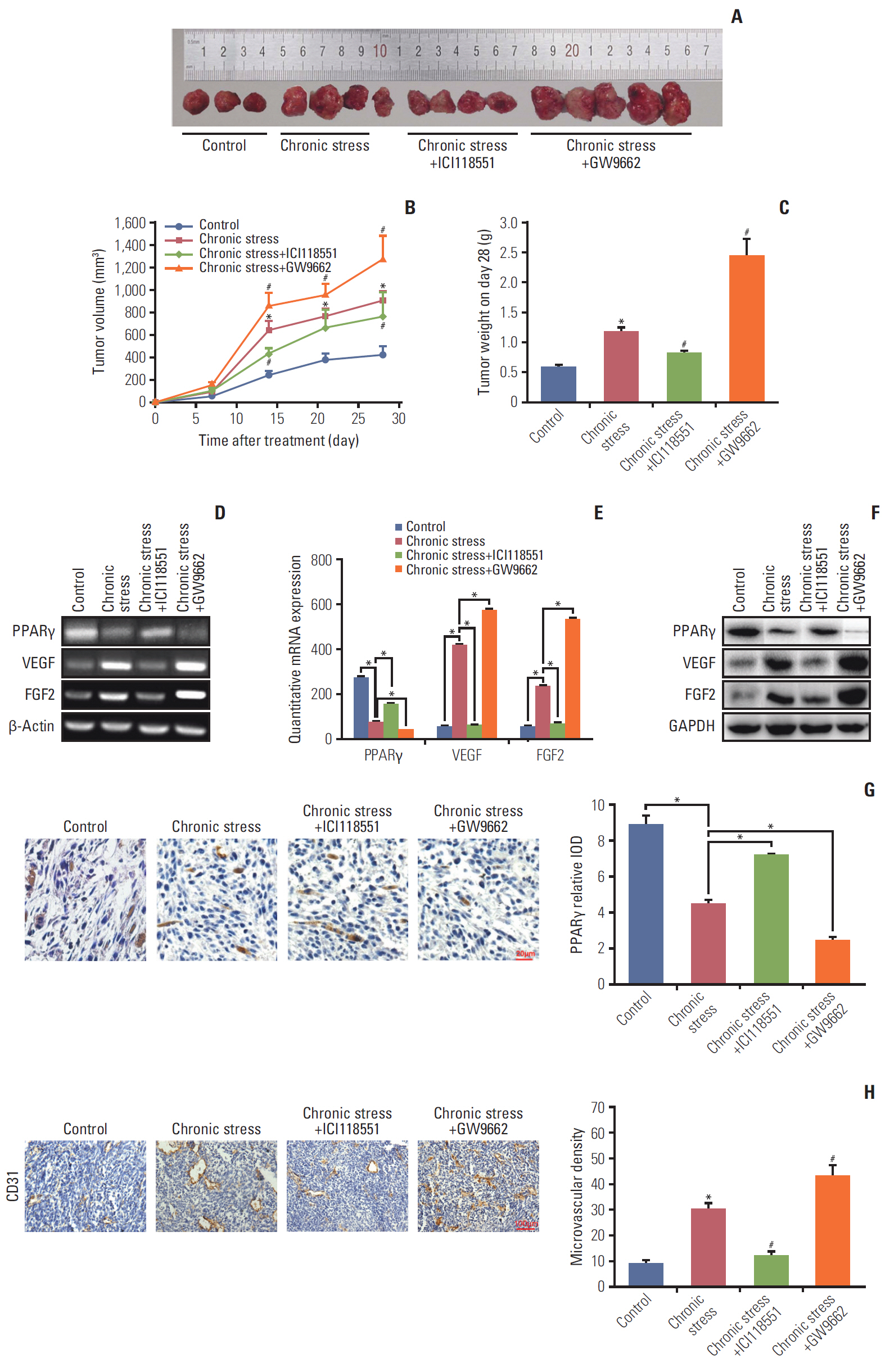
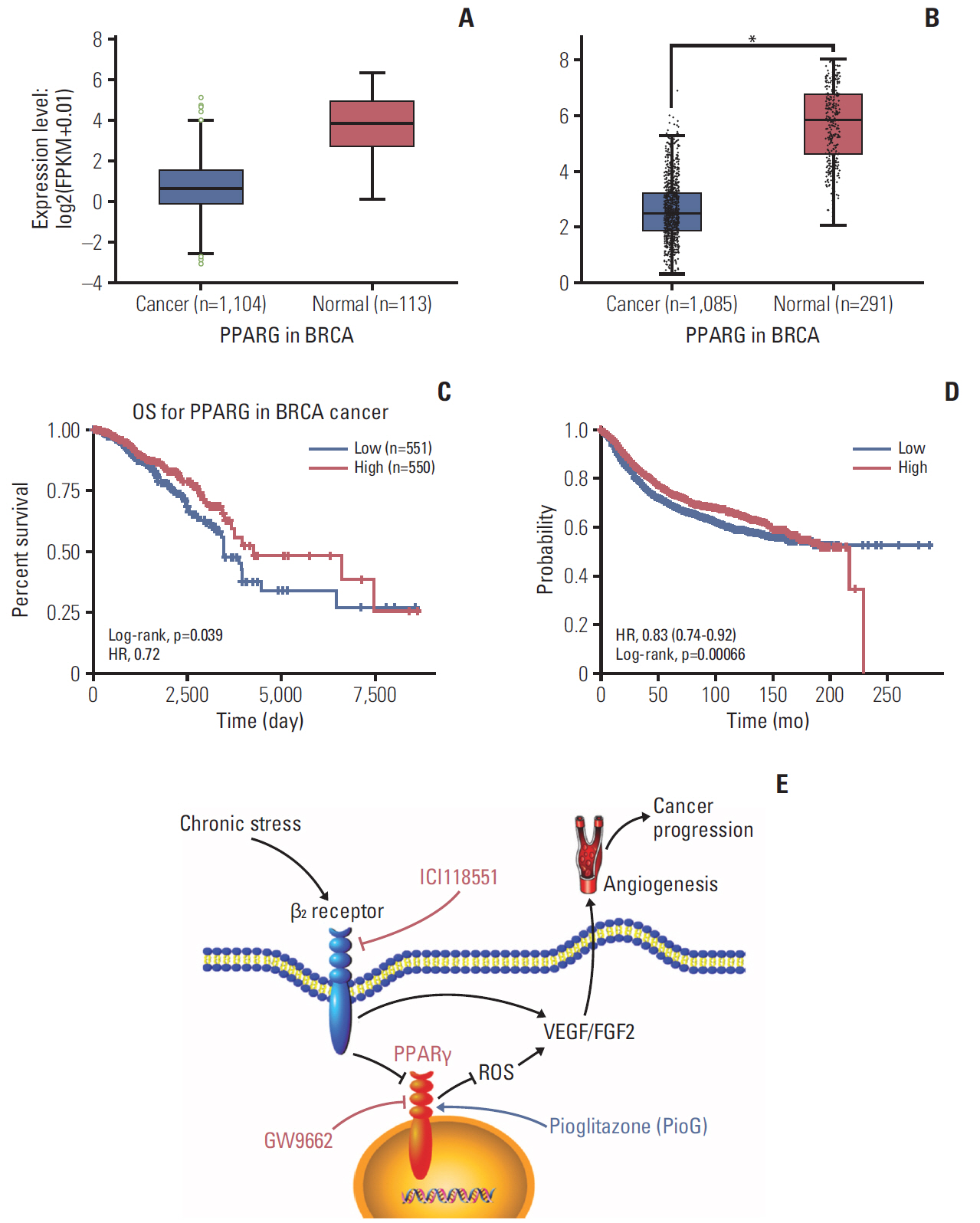
Chronic stress significantly inhibited the PPARγ expression and promoted breast cancer in vivo. VEGF/FGF2-mediated angiogenesis increased in the chronic stress group compared to the control group. PPARγ agonist pioglitazone (PioG) injection offset the pro-tumorigenic effect of chronic stress. Moreover, specific β2-adrenergic receptor (β2R) antagonist ICI11-8551 inhibited the effect of chronic stress. In vitro, norepinephrine (NE) treatment had a similar tendency to chronic stress. The effect of NE was mediated by the β2R/adenylate cyclase signaling pathway and suppressed by PioG. PPARγ suppressed VEGF/FGF2 through reactive oxygen species inhibition. Bioinformatics data confirmed that therewas a lowPPARγ expression in breast invasive carcinoma. Lower PPARγ was associated with a significantly worse survival.
Conclusion
β2R activation induced by chronic stress and related hormones promotes growth and VEGF/FGF2-mediated angiogenesis of breast cancer by down-regulating PPARγ. Our findings hint that β receptor and PPARγ as two target molecules and the novel role for their agonists or antagonists as clinical medicine in breast cancer therapy
Keyword
Figure
Reference
-
References
1. Heidt T, Sager HB, Courties G, Dutta P, Iwamoto Y, Zaltsman A, et al. Chronic variable stress activates hematopoietic stem cells. Nat Med. 2014; 20:754–8.
Article2. Cole SW. Nervous system regulation of the cancer genome. Brain Behav Immun. 2013; 30 Suppl:S10–8.
Article3. Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006; 12:939–44.
Article4. Watkins JL, Thaker PH, Nick AM, Ramondetta LM, Kumar S, Urbauer DL, et al. Clinical impact of selective and nonselective beta-blockers on survival in patients with ovarian cancer. Cancer. 2015; 121:3444–51.
Article5. Nilsson MB, Sun H, Diao L, Tong P, Liu D, Li L, et al. Stress hormones promote EGFR inhibitor resistance in NSCLC: Implications for combinations with beta-blockers. Sci Transl Med. 2017; 9:eaao4307.
Article6. Qin JF, Jin FJ, Li N, Guan HT, Lan L, Ni H, et al. Adrenergic receptor beta2 activation by stress promotes breast cancer progression through macrophages M2 polarization in tumor microenvironment. BMB Rep. 2015; 48:295–300.7. Kim-Fuchs C, Le CP, Pimentel MA, Shackleford D, Ferrari D, Angst E, et al. Chronic stress accelerates pancreatic cancer growth and invasion: a critical role for beta-adrenergic signaling in the pancreatic microenvironment. Brain Behav Immun. 2014; 40:40–7.
Article8. Kwon KA, Yun J, Oh SY, Seo BG, Lee S, Lee JH, et al. Clinical significance of peroxisome proliferator-activated receptor gamma and TRAP220 in patients with operable colorectal cancer. Cancer Res Treat. 2016; 48:198–207.9. Aljada A, O'Connor L, Fu YY, Mousa SA. PPAR gamma ligands, rosiglitazone and pioglitazone, inhibit bFGF- and VEGF-mediated angiogenesis. Angiogenesis. 2008; 11:361–7.10. Shigeto T, Yokoyama Y, Xin B, Mizunuma H. Peroxisome proliferator-activated receptor alpha and gamma ligands inhibit the growth of human ovarian cancer. Oncol Rep. 2007; 18:833–40.11. Messmer D, Lorrain K, Stebbins K, Bravo Y, Stock N, Cabrera G, et al. A selective novel peroxisome proliferator-activated receptor (PPAR)-alpha antagonist induces apoptosis and inhibits proliferation of CLL cells in vitro and in vivo. Mol Med. 2015; 21:410–9.12. Hong OY, Youn HJ, Jang HY, Jung SH, Noh EM, Chae HS, et al. Troglitazone inhibits matrix metalloproteinase-9 expression and invasion of breast cancer cell through a peroxisome proliferator-activated receptor gamma-dependent mechanism. J Breast Cancer. 2018; 21:28–36.13. Lindgren EM, Nielsen R, Petrovic N, Jacobsson A, Mandrup S, Cannon B, et al. Noradrenaline represses PPAR (peroxisome-proliferator-activated receptor) gamma2 gene expression in brown adipocytes: intracellular signalling and effects on PPARgamma2 and PPARgamma1 protein levels. Biochem J. 2004; 382:597–606.14. Guo M, Li C, Lei Y, Xu S, Zhao D, Lu XY. Role of the adipose PPARgamma-adiponectin axis in susceptibility to stress and depression/anxiety-related behaviors. Mol Psychiatry. 2017; 22:1056–68.15. Huber S, Valente S, Chaimbault P, Schohn H. Evaluation of ∆2-pioglitazone, an analogue of pioglitazone, on colon cancer cell survival: evidence of drug treatment association with autophagy and activation of the Nrf2/Keap1 pathway. Int J Oncol. 2014; 45:426–38.
Article16. Hsiao PJ, Chiou HC, Jiang HJ, Lee MY, Hsieh TJ, Kuo KK. Pioglitazone enhances cytosolic lipolysis, beta-oxidation and autophagy to ameliorate hepatic steatosis. Sci Rep. 2017; 7:9030.
Article17. Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000; 407:249–57.
Article18. Lutgendorf SK, Cole S, Costanzo E, Bradley S, Coffin J, Jabbari S, et al. Stress-related mediators stimulate vascular endothelial growth factor secretion by two ovarian cancer cell lines. Clin Cancer Res. 2003; 9:4514–21.19. Srivastava N, Kollipara RK, Singh DK, Sudderth J, Hu Z, Nguyen H, et al. Inhibition of cancer cell proliferation by PPARgamma is mediated by a metabolic switch that increases reactive oxygen species levels. Cell Metab. 2014; 20:650–61.20. Ushio-Fukai M. Redox signaling in angiogenesis: role of NADPH oxidase. Cardiovasc Res. 2006; 71:226–35.21. Li JH, Liu S, Zhou H, Qu LH, Yang JH. starBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2014; 42:D92–7.
Article22. Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017; 45:W98–102.
Article23. Qin J, Liu H, Li Y, Liu B, Ni H. Effect of hormones from adrenal gland on breast cancer metastasis in mice. Cancer Res Prev Treat. 2016; 43:1013–7.24. Nasir A, Bullo MM, Ahmed Z, Imtiaz A, Yaqoob E, Jadoon M, et al. Nutrigenomics: epigenetics and cancer prevention: a comprehensive review. Crit Rev Food Sci Nutr. 2020; 60:1375–87.
Article25. Hill EM, Watkins K. Women with ovarian cancer: examining the role of social support and rumination in posttraumatic growth, psychological distress, and psychological well-being. J Clin Psychol Med Settings. 2017; 24:47–58.
Article26. Botteri E, Munzone E, Rotmensz N, Cipolla C, De Giorgi V, Santillo B, et al. Therapeutic effect of beta-blockers in triple-negative breast cancer postmenopausal women. Breast Cancer Res Treat. 2013; 140:567–75.27. Wallukat G. The beta-adrenergic receptors. Herz. 2002; 27:683–90.28. Chen HY, Liu Q, Salter AM, Lomax MA. Synergism between cAMP and PPARgamma signalling in the initiation of UCP1 gene expression in HIB1B brown adipocytes. PPAR Res. 2013; 2013:476049.29. Luz AL, Kassotis CD, Stapleton HM, Meyer JN. The high-production volume fungicide pyraclostrobin induces triglyceride accumulation associated with mitochondrial dysfunction, and promotes adipocyte differentiation independent of PPARgamma activation, in 3T3-L1 cells. Toxicology. 2018; 393:150–9.30. Pineda-Belmontes CP, Hernandez-Ramirez RU, Hernandez-Alcaraz C, Cebrian ME, Lopez-Carrillo L. Genetic polymorphisms of PPAR gamma, arsenic methylation capacity and breast cancer risk in Mexican women. Salud Publica Mex. 2016; 58:220–7.
Article31. Giaginis C, Politi E, Alexandrou P, Sfiniadakis J, Kouraklis G, Theocharis S. Expression of peroxisome proliferator activated receptor-gamma (PPAR-gamma) in human non-small cell lung carcinoma: correlation with clinicopathological parameters, proliferation and apoptosis related molecules and patients' survival. Pathol Oncol Res. 2012; 18:875–83.32. Lee EJ, Park JS, Lee YY, Kim DY, Kang JL, Kim HS. Antiinflammatory and anti-oxidant mechanisms of an MMP-8 inhibitor in lipoteichoic acid-stimulated rat primary astrocytes: involvement of NF-kappaB, Nrf2, and PPAR-gamma signaling pathways. J Neuroinflammation. 2018; 15:326.
Article33. Amor S, Iglesias-de la Cruz MC, Ferrero E, Garcia-Villar O, Barrios V, Fernandez N, et al. Peritumoral adipose tissue as a source of inflammatory and angiogenic factors in colorectal cancer. Int J Colorectal Dis. 2016; 31:365–75.
Article34. Yoshizaki T, Motomura W, Tanno S, Kumei S, Yoshizaki Y, Tanno S, et al. Thiazolidinediones enhance vascular endothelial growth factor expression and induce cell growth inhibition in non-small-cell lung cancer cells. J Exp Clin Cancer Res. 2010; 29:22.
Article35. Chiu M, McBeth L, Sindhwani P, Hinds TD. Deciphering the roles of thiazolidinediones and PPARgamma in bladder cancer. PPAR Res. 2017; 2017:4810672.36. Moloney JN, Cotter TG. ROS signalling in the biology of cancer. Semin Cell Dev Biol. 2018; 80:50–64.
Article37. Majumder A, Singh M, George AK, Behera J, Tyagi N, Tyagi SC. Hydrogen sulfide improves postischemic neoangiogenesis in the hind limb of cystathionine-beta-synthase mutant mice via PPAR-gamma/VEGF axis. Physiol Rep. 2018; 6:e13858.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Norepinephrine/β2 -Adrenergic Receptor Pathway Promotes the Cell Proliferation and Nerve Growth Factor Production in Triple-Negative Breast Cancer
- Expression of Cyclooxygenase-2 in Human Breast Carcinoma: Relevance to Tumor Angiogenesis and Expression of Estrogen Receptor
- Isomangiferin, a Novel Potent Vascular Endothelial Growth Factor Receptor 2 Kinase Inhibitor, Suppresses Breast Cancer Growth, Metastasis and Angiogenesis
- Invited Commentary: Role of Estrogen Receptor-alpha in Regulating Claudin-6 Expression in Breast Cancer Cells
- The Relationship between PTEN Tumor Suppressor Gene and Vascular Endothelial Growth Factor-Mediated Angiogenesis in Breast Cancer