Int J Stem Cells.
2020 Jul;13(2):268-278. 10.15283/ijsc20028.
Adipose Tissue-Derived Stem Cells from Type 2 Diabetics Reveal Conservative Alterations in Multidimensional Characteristics
- Affiliations
-
- 1Organ Transplant Center, Tianjin First Central Hospital, Nankai University, Tianjin, China
- 2NHC Key Laboratory for Critical Care Medicine, Tianjin, China
- 3Tianjin Clinical Research Center for Organ Transplantation, Tianjin, China
- 4The Postdoctoral Research Station, School of Medicine, Nankai University, Tianjin, China
- KMID: 2504339
- DOI: http://doi.org/10.15283/ijsc20028
Abstract
- Background and Objectives
Adipose tissue-derived mesenchymal stem cells (ASCs) are recognized as an advantaged source for the prevention and treatment of diverse diseases including type 2 diabetes mellitus (T2DM). However, alterations in characteristics of ASCs from the aforementioned T2DM patients are still obscure, which also hinder the rigorous and systematic illumination of progression and pathogenesis.
Methods and Results
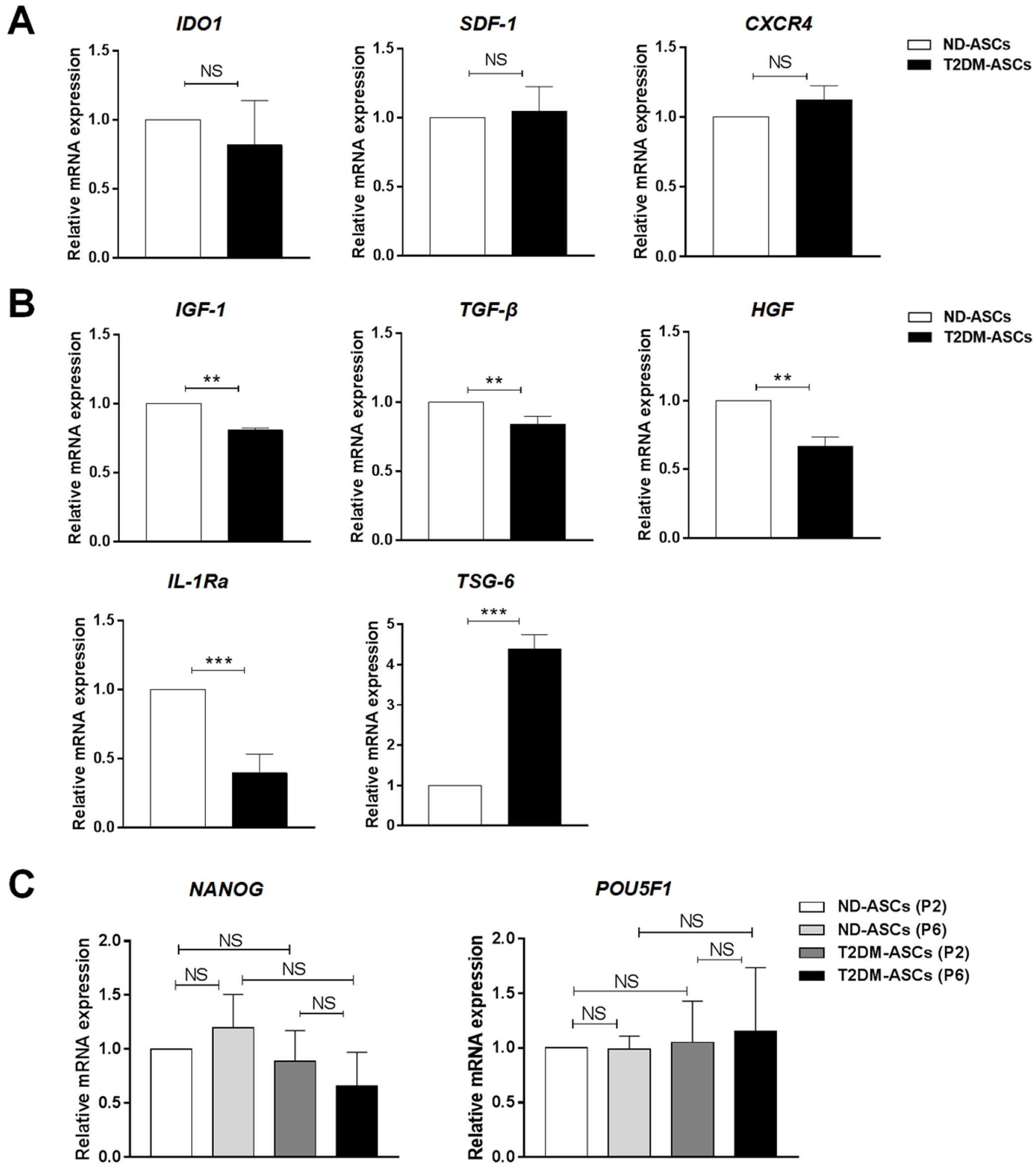
In this study, we originally isolated peripancreatic adipose tissue-derived mesenchymal stem cells from both human type 2 diabetic and non-diabetic donors (T2DM-ASCs, ND-ASCs) with the parental consent, respectively. We noticed that T2DM-ASCs exhibited indistinguishable immunophenotype, cell vitality, chondrogenic differentiation and stemness as ND-ASCs. Simultaneously, there’s merely alterations in migration and immunoregulatory capacities in T2DM-ASCs. However, differing from ND-ASCs, T2DM-ASCs exhibited deficiency in adipogenic and osteogenic differentiation, and in particular, the delayed cell cycle and different cytokine expression spectrum.
Conclusions
The conservative alterations of T2DM-ASCs in multifaceted characteristics indicated the possibility of autologous application of ASCs for cell-based T2DM treatment in the future.
Keyword
Figure
Reference
-
References
1. Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J, Shan Z, Liu J, Tian H, Ji Q, Zhu D, Ge J, Lin L, Chen L, Guo X, Zhao Z, Li Q, Zhou Z, Shan G, He J. China National Diabetes and Metabolic Disorders Study Group. 2010; Prevalence of diabetes among men and women in China. N Engl J Med. 362:1090–1101. DOI: 10.1056/NEJMoa0908292. PMID: 20335585.
Article2. He X, Yang Y, Yao MW, Ren TT, Guo W, Li L, Xu X. 2019; Full title: High glucose protects mesenchymal stem cells from metformin-induced apoptosis through the AMPK-mediated mTOR pathway. Sci Rep. 9:17764. DOI: 10.1038/s41598-019-54291-y. PMID: 31780804. PMCID: PMC6882892.
Article3. Zang L, Hao H, Liu J, Li Y, Han W, Mu Y. 2017; Mesenchymal stem cell therapy in type 2 diabetes mellitus. Diabetol Metab Syndr. 9:36. DOI: 10.1186/s13098-017-0233-1. PMID: 28515792. PMCID: PMC5433043.
Article4. Cho J, D'Antuono M, Glicksman M, Wang J, Jonklaas J. 2018; A review of clinical trials: mesenchymal stem cell transplant therapy in type 1 and type 2 diabetes mellitus. Am J Stem Cells. 7:82–93. PMID: 30510843. PMCID: PMC6261870.5. Qi Y, Ma J, Li S, Liu W. 2019; Applicability of adipose-derived mesenchymal stem cells in treatment of patients with type 2 diabetes. Stem Cell Res Ther. 10:274. DOI: 10.1186/s13287-019-1362-2. PMID: 31455405. PMCID: PMC6712852.
Article6. Ying W, Fu W, Lee YS, Olefsky JM. 2020; The role of macrophages in obesity-associated islet inflammation and β-cell abnormalities. Nat Rev Endocrinol. 16:81–90. DOI: 10.1038/s41574-019-0286-3. PMID: 31836875.
Article7. Holmes D. 2014; Diabetes: MSC transplant prevents β-cell dysfunction. Nat Rev Endocrinol. 10:701. DOI: 10.1038/nrendo.2014.172. PMID: 25265979.8. Samsonraj RM, Raghunath M, Nurcombe V, Hui JH, van Wijnen AJ, Cool SM. 2017; Concise review: multifaceted characterization of human mesenchymal stem cells for use in regenerative medicine. Stem Cells Transl Med. 6:2173–2185. DOI: 10.1002/sctm.17-0129. PMID: 29076267. PMCID: PMC5702523.
Article9. Zhang L, Wang H, Liu C, Wu Q, Su P, Wu D, Guo J, Zhou W, Xu Y, Shi L, Zhou J. 2018; MSX2 Initiates and accelerates mesenchymal stem/stromal cell specification of hPSCs by regulating TWIST1 and PRAME. Stem Cell Reports. 11:497–513. DOI: 10.1016/j.stemcr.2018.06.019. PMID: 30033084. PMCID: PMC6092836.
Article10. Zhao Q, Zhang L, Wei Y, Yu H, Zou L, Huo J, Yang H, Song B, Wei T, Wu D, Zhang W, Zhang L, Liu D, Li Z, Chi Y, Han Z, Han Z. 2019; Systematic comparison of hUC-MSCs at various passages reveals the variations of signatures and therapeutic effect on acute graft-versus-host disease. Stem Cell Res Ther. 10:354. DOI: 10.1186/s13287-019-1478-4. PMID: 31779707. PMCID: PMC6883552.
Article11. Kabat M, Bobkov I, Kumar S, Grumet M. 2020; Trends in mesenchymal stem cell clinical trials 2004-2018: is efficacy optimal in a narrow dose range? Stem Cells Transl Med. 9:17–27. DOI: 10.1002/sctm.19-0202. PMID: 31804767. PMCID: PMC6954709.
Article12. Shi Y, Wang Y, Li Q, Liu K, Hou J, Shao C, Wang Y. 2018; Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol. 14:493–507. DOI: 10.1038/s41581-018-0023-5. PMID: 29895977.
Article13. Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. 1968; Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 6:230–247. DOI: 10.1097/00007890-196803000-00009. PMID: 5654088.14. Wei Y, Hou H, Zhang L, Zhao N, Li C, Huo J, Liu Y, Zhang W, Li Z, Liu D, Han Z, Zhang L, Song B, Chi Y, Han Z. 2019; JNKi- and DAC-programmed mesenchymal stem/stromal cells from hESCs facilitate hematopoiesis and alleviate hind limb ischemia. Stem Cell Res Ther. 10:186. DOI: 10.1186/s13287-019-1302-1. PMID: 31234947. PMCID: PMC6591900.
Article15. Zhang X, Yang Y, Zhang L, Lu Y, Zhang Q, Fan D, Zhang Y, Zhang Y, Ye Z, Xiong D. 2017; Mesenchymal stromal cells as vehicles of tetravalent bispecific Tandab (CD3/CD19) for the treatment of B cell lymphoma combined with IDO pathway inhibitor D-1-methyl-tryptophan. J Hematol Oncol. 10:56. DOI: 10.1186/s13045-017-0397-z. PMID: 28228105. PMCID: PMC5322661.
Article16. Yao J, Chen N, Wang X, Zhang L, Huo J, Chi Y, Li Z, Han Z. 2020; Human supernumerary teeth-derived apical papillary stem cells possess preferable characteristics and efficacy on hepatic fibrosis in mice. Stem Cells Int. 2020:6489396. DOI: 10.1155/2020/6489396. PMID: 32399047. PMCID: PMC7204141.
Article17. Pourgholaminejad A, Aghdami N, Baharvand H, Moazzeni SM. 2016; The effect of pro-inflammatory cytokines on immunophenotype, differentiation capacity and immunomodulatory functions of human mesenchymal stem cells. Cytokine. 85:51–60. DOI: 10.1016/j.cyto.2016.06.003. PMID: 27288632.
Article18. McGonagle D, Baboolal TG, Jones E. 2017; Native joint-resident mesenchymal stem cells for cartilage repair in osteoarthritis. Nat Rev Rheumatol. 13:719–730. DOI: 10.1038/nrrheum.2017.182. PMID: 29118440.
Article19. Kfoury Y, Scadden DT. 2015; Mesenchymal cell contributions to the stem cell niche. Cell Stem Cell. 16:239–253. DOI: 10.1016/j.stem.2015.02.019. PMID: 25748931.
Article20. Nombela-Arrieta C, Ritz J, Silberstein LE. 2011; The elusive nature and function of mesenchymal stem cells. Nat Rev Mol Cell Biol. 12:126–131. DOI: 10.1038/nrm3049. PMID: 21253000. PMCID: PMC3346289.
Article21. He Y, Xu LL, Feng FE, Wang QM, Zhu XL, Wang CC, Zhang JM, Fu HX, Xu LP, Liu KY, Huang XJ, Zhang XH. 2018; Mesenchymal stem cell deficiency influences megakaryocytopoiesis through the TNFAIP3/NF-κB/SMAD pathway in patients with immune thrombocytopenia. Br J Haematol. 180:395–411. DOI: 10.1111/bjh.15034. PMID: 29327472.
Article22. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. 2003; Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 112:1796–1808. DOI: 10.1172/JCI200319246. PMID: 14679176. PMCID: PMC296995.
Article23. Wang L, Liu T, Liang R, Wang G, Liu Y, Zou J, Liu N, Zhang B, Liu Y, Ding X, Cai X, Wang Z, Xu X, Ricordi C, Wang S, Shen Z. 2020; Mesenchymal stem cells ameliorate β cell dysfunction of human type 2 diabetic islets by reversing β cell dedifferentiation. EBioMedicine. 51:102615. DOI: 10.1016/j.ebiom.2019.102615. PMID: 31918404. PMCID: PMC7000334.24. Kočí Z, Turnovcová K, Dubský M, Baranovičová L, Holáň V, Chudíčková M, Syková E, Kubinová S. 2014; Characterization of human adipose tissue-derived stromal cells isolated from diabetic patient's distal limbs with critical ischemia. Cell Biochem Funct. 32:597–604. DOI: 10.1002/cbf.3056. PMID: 25251698.
Article25. Barbagallo I, Li Volti G, Galvano F, Tettamanti G, Pluchinotta FR, Bergante S, Vanella L. 2017; Diabetic human adipose tissue-derived mesenchymal stem cells fail to differentiate in functional adipocytes. Exp Biol Med (Maywood). 242:1079–1085. DOI: 10.1177/1535370216681552. PMID: 27909015. PMCID: PMC5444636.
Article26. Alicka M, Major P, Wysocki M, Marycz K. 2019; Adipose-derived mesenchymal stem cells isolated from patients with type 2 diabetes show reduced "stemness" through an altered secretome profile, impaired anti-oxidative protection, and mitochondrial dynamics deterioration. J Clin Med. 8:765. DOI: 10.3390/jcm8060765. PMID: 31151180. PMCID: PMC6617220.
Article27. Lim M, Wang W, Liang L, Han ZB, Li Z, Geng J, Zhao M, Jia H, Feng J, Wei Z, Song B, Zhang J, Li J, Liu T, Wang F, Li T, Li J, Fang Y, Gao J, Han Z. 2018; Intravenous injection of allogeneic umbilical cord-derived multipotent mesenchymal stromal cells reduces the infarct area and ameliorates cardiac function in a porcine model of acute myocardial infarction. Stem Cell Res Ther. 9:129. DOI: 10.1186/s13287-018-0888-z. PMID: 29751831. PMCID: PMC5948807.
Article28. Zhang L, Liu C, Wang H, Wu D, Su P, Wang M, Guo J, Zhao S, Dong S, Zhou W, Arakaki C, Zhang X, Zhou J. 2018; Thrombopoietin knock-in augments platelet generation from human embryonic stem cells. Stem Cell Res Ther. 9:194. DOI: 10.1186/s13287-018-0926-x. PMID: 30016991. PMCID: PMC6050740.
Article29. Pachón-Peña G, Serena C, Ejarque M, Petriz J, Duran X, Oliva-Olivera W, Simó R, Tinahones FJ, Fernández-Veledo S, Vendrell J. 2016; Obesity determines the immunophenotypic profile and functional characteristics of human mesenchymal stem cells from adipose tissue. Stem Cells Transl Med. 5:464–475. DOI: 10.5966/sctm.2015-0161. PMID: 26956208. PMCID: PMC4798735.
Article30. Serena C, Keiran N, Ceperuelo-Mallafre V, Ejarque M, Fradera R, Roche K, Nuñez-Roa C, Vendrell J, Fernández-Veledo S. 2016; Obesity and type 2 diabetes alters the immune properties of human adipose derived stem cells. Stem Cells. 34:2559–2573. DOI: 10.1002/stem.2429. PMID: 27352919.
Article31. Kornicka K, Houston J, Marycz K. 2018; Dysfunction of mesenchymal stem cells isolated from metabolic syndrome and type 2 diabetic patients as result of oxidative stress and autophagy may limit their potential therapeutic use. Stem Cell Rev Rep. 14:337–345. DOI: 10.1007/s12015-018-9809-x. PMID: 29611042. PMCID: PMC5960487.
Article32. Hankamolsiri W, Manochantr S, Tantrawatpan C, Tantikanlayaporn D, Tapanadechopone P, Kheolamai P. 2016; The effects of high glucose on adipogenic and osteogenic differentiation of gestational tissue-derived MSCs. Stem Cells Int. 2016:9674614. DOI: 10.1155/2016/9674614. PMID: 27057179. PMCID: PMC4707328.
Article33. Lafosse A, Dufeys C, Beauloye C, Horman S, Dufrane D. 2016; Impact of hyperglycemia and low oxygen tension on adipose-derived stem cells compared with dermal fibroblasts and keratinocytes: importance for wound healing in type 2 diabetes. PLoS One. 11:e0168058. DOI: 10.1371/journal.pone.0168058. PMID: 27992567. PMCID: PMC5167273.
Article34. Krishnan B, Sallam HS, Tumurbataar B, Saieva S, Baymon D, Tuvdendorj D, Micci MA, Abate N, Taglialatela G. 2020; Amelioration of hippocampal dysfunction by adipose tissue-targeted stem cell transplantation in a mouse model of type 2 diabetes. J Neurochem. 153:51–62. DOI: 10.1111/jnc.14915. PMID: 31730234.
Article35. Qi K, Li N, Zhang Z, Melino G. 2018; Tissue regeneration: the crosstalk between mesenchymal stem cells and immune response. Cell Immunol. 326:86–93. DOI: 10.1016/j.cellimm.2017.11.010. PMID: 29221689.
Article36. Caplan AI. 2017; Mesenchymal stem cells: time to change the name! Stem Cells Transl Med. 6:1445–1451. DOI: 10.1002/sctm.17-0051. PMID: 28452204. PMCID: PMC5689741.
Article37. Bernardo ME, Fibbe WE. 2013; Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell. 13:392–402. DOI: 10.1016/j.stem.2013.09.006. PMID: 24094322.
Article38. Cornu M, Thorens B. 2009; GLP-1 protects β-cells against apoptosis by enhancing the activity of an IGF-2/IGF1-receptor autocrine loop. Islets. 1:280–282. DOI: 10.4161/isl.1.3.9932. PMID: 21099285.
Article39. Gaddy DF, Riedel MJ, Pejawar-Gaddy S, Kieffer TJ, Robbins PD. 2010; In vivo expression of HGF/NK1 and GLP-1 from dsAAV vectors enhances pancreatic ß-cell proliferation and improves pathology in the db/db mouse model of diabetes. Diabetes. 59:3108–3116. DOI: 10.2337/db09-1886. PMID: 20841608. PMCID: PMC2992772.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Adipose-derived stem cells: characterization and clinical application
- Adipose Tissue - Adequate, Accessible Regenerative Material
- Adipose Tissue Derived Mesenchymal Stem Cells
- Concise Review: Differentiation of Human Adult Stem Cells Into Hepatocyte-like Cells In vitro
- Brief Review of Adipose Derived Cells