J Rhinol.
2020 May;27(1):28-33. 10.18787/jr.2019.00297.
Sphenoid Sinus Fat Packing in Transsphenoidal Surgery: Long-Term Fate Assessment Using Magnetic Resonance Imaging
- Affiliations
-
- 1Department of Otorhinolaryngology and 2Neurosurgery, Yonsei University Wonju College of Medicine, Wonju, Korea
- 2Yonsei University Wonju College of Medicine, Wonju, Korea
- KMID: 2502791
- DOI: http://doi.org/10.18787/jr.2019.00297
Abstract
- Background and Objectives
Following the transsphenoidal approach (TSA), appropriate sphenoid sinus fat packing has been preferred to prevent postoperative cerebrospinal fluid leakage; however, studies on the behavior of fat tissue transplanted in the sphenoid sinus are lacking. This study aimed to determine the long-term fate of these fat grafts using magnetic resonance imaging (MRI).
Subjects and Method
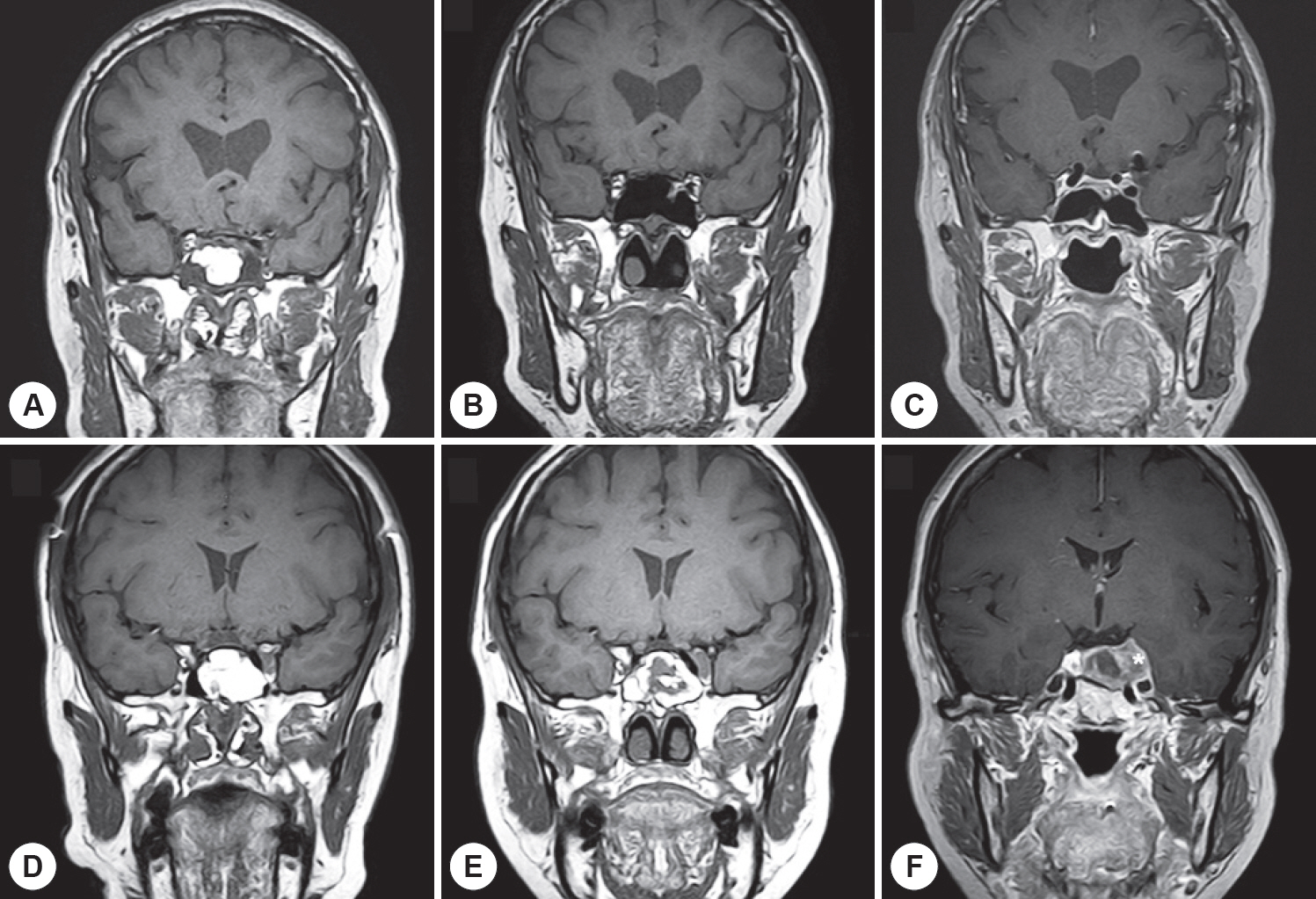
A total of 139 postoperative MRI scans of 41 patients who underwent sphenoid sinus fat packing using the standard TSA were evaluated. Additionally, MRI time series indicating the vital fat volumes were assessed postoperatively.
Results
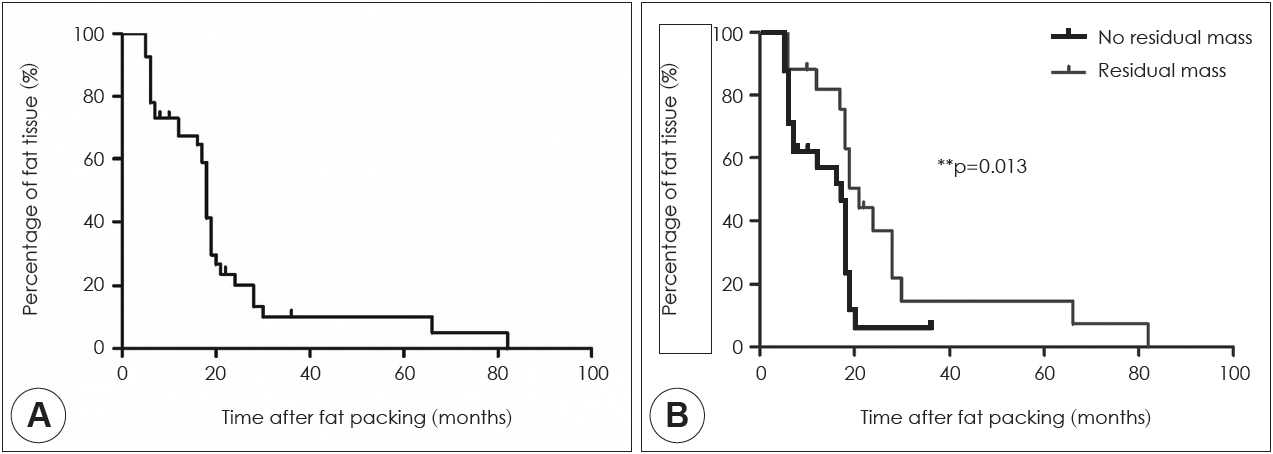
In 82.9% of cases, the fat volumes measured in the final MRI scans declined to <20% of the initial volumes; only 4.9% of cases exhibited declines to >60% of the initial volume. The fat tissue volume decreased significantly with time, with a median half-life of 18 months. Typically, the sphenoid sinus was eventually almost filled with air rather than transplanted fat. In the subgroup analysis, the fat clearance rate was significantly lower in patients with residual tumors than in those without such remnants (p=0.013).
Conclusion
Long-term MRI surveillance of fat grafts in the sphenoid sinus revealed that the transplanted fat graft had degraded and was gradually eliminated.
Keyword
Figure
Reference
-
1. Ezzat S, Asa SL, Couldwell WT, Barr CE, Dodge WE, Vance ML, et al. The prevalence of pituitary adenomas: a systematic review. Cancer. 2004; 101:613–9.2. Molitch ME. Nonfunctioning pituitary tumors. Handb Clin Neurol. 2014; 124:167–84.3. Sonnenburg RE, White D, Ewend MG, Senior B. The learning curve in minimally invasive pituitary surgery. Am J Rhinol. 2004; 18:25963.4. Arita K, Kurisu K, Tominaga A, Ikawa F, Iida K, Hama S, et al. Sizeadjustable titanium plate for reconstruction of the sella turcica. Technical note. J Neurosurg. 1999; 91:1055–7.5. Cappabianca P, Cavallo LM, Mariniello G, de Divitiis O, Romero AD, de Divitiis E. Easy sellar reconstruction in endoscopic endonasal transsphenoidal surgery with polyester-silicone dural substitute and fibrin glue: technical note. Neurosurgery. 2001; 49:473–5. discussion 5-6.6. Couldwell WT, Kan P, Weiss MH. Simple closure following transsphenoidal surgery. Technical note. Neurosurg Focus. 2006; 20:E11.7. Chen HC, Lee ST. Need for intrasellar packing in sellar reconstruction of transsphenoidal surgery: less is more? J Clin Neurosci. 2006; 13:423–7.8. Seda L, Camara RB, Cukiert A, Burattini JA, Mariani PP. Sellar floor reconstruction after transsphenoidal surgery using fibrin glue without grafting or implants: technical note. Surg Neurol. 2006; 66:46–9. discussion 9.9. Farrell CJ, Nyquist GG, Farag AA, Rosen MR, Evans JJ. Principles of Pituitary Surgery. Otolaryngol Clin North Am. 2016; 49:95–106.10. Sinno S, Wilson S, Brownstone N, Levine SM. Current Thoughts on Fat Grafting: Using the Evidence to Determine Fact or Fiction. Plast Reconstr Surg. 2016; 137:818–24.11. Sezgin B, Ozmen S, Bulam H, Omeroglu S, Yuksel S, Cayci B, et al. Improving fat graft survival through preconditioning of the recipient site with microneedling. J Plast Reconstr Aesthet Surg. 2014; 67:712–20.12. Doornaert M, Colle J, De Maere E, Declercq H, Blondeel P. Autologous fat grafting: Latest insights. Ann Med Surg (Lond). 2019; 37:47–53.13. Carpaneda CA, Ribeiro MT. Percentage of graft viability versus injected volume in adipose autotransplants. Aesthetic Plast Surg. 1994; 18:17–9.14. Fatemi N, Dusick JR, de Paiva Neto MA, Kelly DF. The endonasal microscopic approach for pituitary adenomas and other parasellar tumors: a 10-year experience. Neurosurgery. 2008; 63:244–56. discussion 56.15. Roca E, Penn DL, Safain MG, Burke WT, Castlen JP, Laws ER Jr. Abdominal Fat Graft for Sellar Reconstruction: Retrospective Outcomes Review and Technical Note. Oper Neurosurg (Hagerstown). 2018.16. Weber R, Draf W, Keerl R, Kahle G, Schinzel S, Thomann S, et al. Osteoplastic frontal sinus surgery with fat obliteration: technique and long-term results using magnetic resonance imaging in 82 operations. Laryngoscope. 2000; 110:1037–44.17. Tien RD. Fat-suppression MR imaging in neuroradiology: techniques and clinical application. AJR Am J Roentgenol. 1992; 158:369–79.18. Hansen FS, van der Poel NA, Freling NJM, Fokkens WJ. Mucocele formation after frontal sinus obliteration. Rhinology. 2018.19. Gir P, Brown SA, Oni G, Kashefi N, Mojallal A, Rohrich RJ. Fat grafting: evidence-based review on autologous fat harvesting, processing, reinjection, and storage. Plast Reconstr Surg. 2012; 130:24958.20. Eloy JA, Marchiano E, Vazquez A. Extended Endoscopic and Open Sinus Surgery for Refractory Chronic Rhinosinusitis. Otolaryngol Clin North Am. 2017; 50:165–82.21. Constantinidis J, Bohr C, Greess H, Aigner T, Zenk J, Prokopakis E, et al. Fat obliteration in paranasal sinuses: a comparative magnetic resonance imaging and histopathologic study. Laryngoscope. 2005; 115:717–23.22. Shiffman MA, Mirrafati S. Fat transfer techniques: the effect of harvest and transfer methods on adipocyte viability and review of the literature. Dermatol Surg. 2001; 27:819–26.23. Santos CR, Schulze A. Lipid metabolism in cancer. FEBS J. 2012; 279:2610–23.24. Porstmann T, Santos CR, Griffiths B, Cully M, Wu M, Leevers S, et al. SREBP activity is regulated by mTORC1 and contributes to Aktdependent cell growth. Cell Metab. 2008; 8:224–36.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Endoscopic Endonasal Transsphenoidal Resection of Solitary Extramedullary Plasmacytoma in the Sphenoid Sinus with Destruction of Skull Base
- Pnumatization of the Sphenoid Sinus in Children Evaluated by Magnetic Resonance Imaging
- A Case of Optic Neuropathy Caused by Sphenoid Sinus Mucocele
- New Landmark for the Endoscopic Endonasal Transsphenoidal Approach of Pituitary Surgery
- A Case of Pituitary Metastasis of Lung Cancer Presenting as Cavernous Sinus Syndrome