Korean J Physiol Pharmacol.
2020 May;24(3):203-212. 10.4196/kjpp.2020.24.3.203.
Interaction of promyelocytic leukemia/p53 affects signal transducer and activator of transcription-3 activity in response to oncostatin M
- Affiliations
-
- 1Departments of Physiology, Ewha Womans University College of Medicine, Seoul 07804, Korea
- 2Departments of Pharmacology, Ewha Womans University College of Medicine, Seoul 07804, Korea
- KMID: 2502502
- DOI: http://doi.org/10.4196/kjpp.2020.24.3.203
Abstract
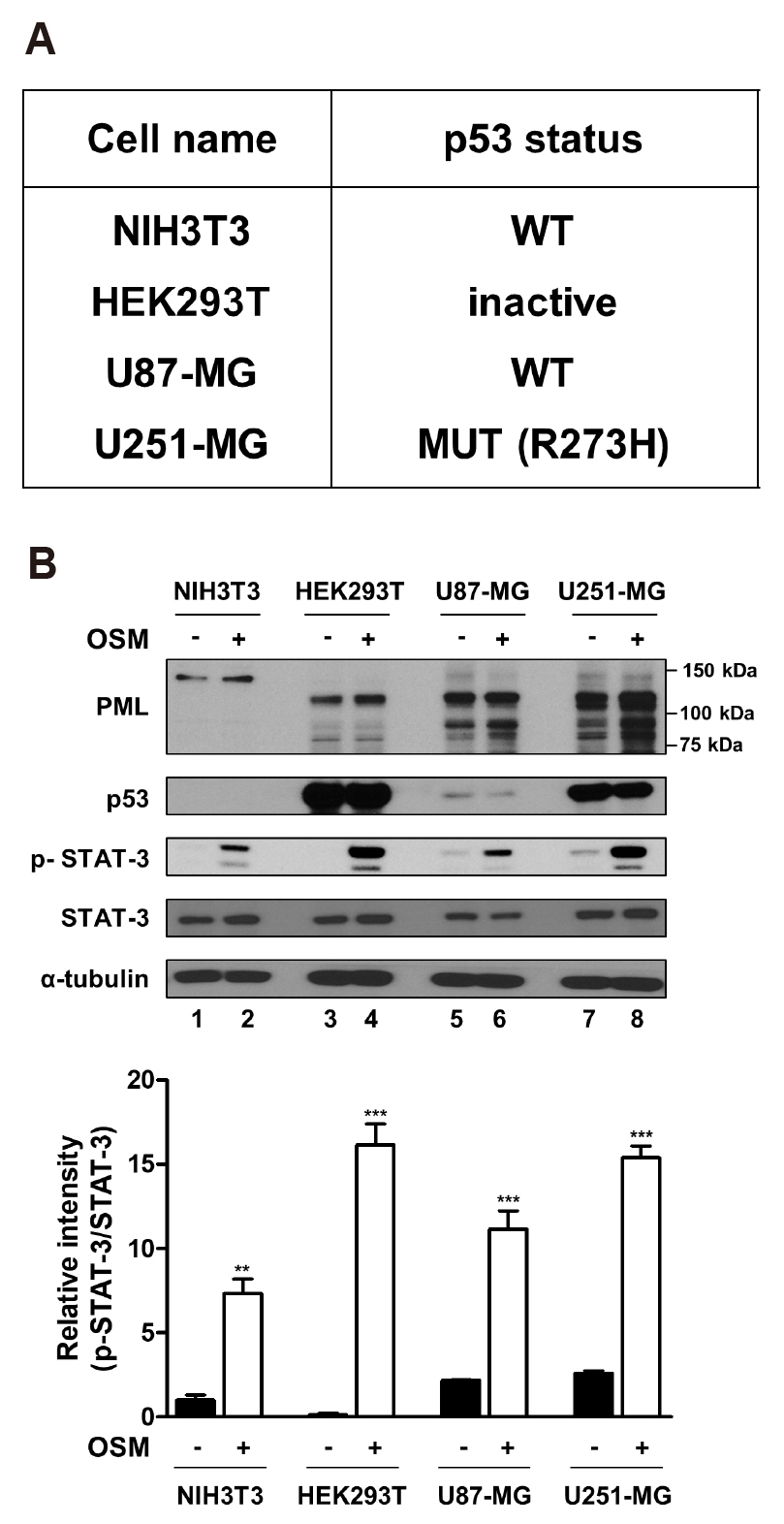
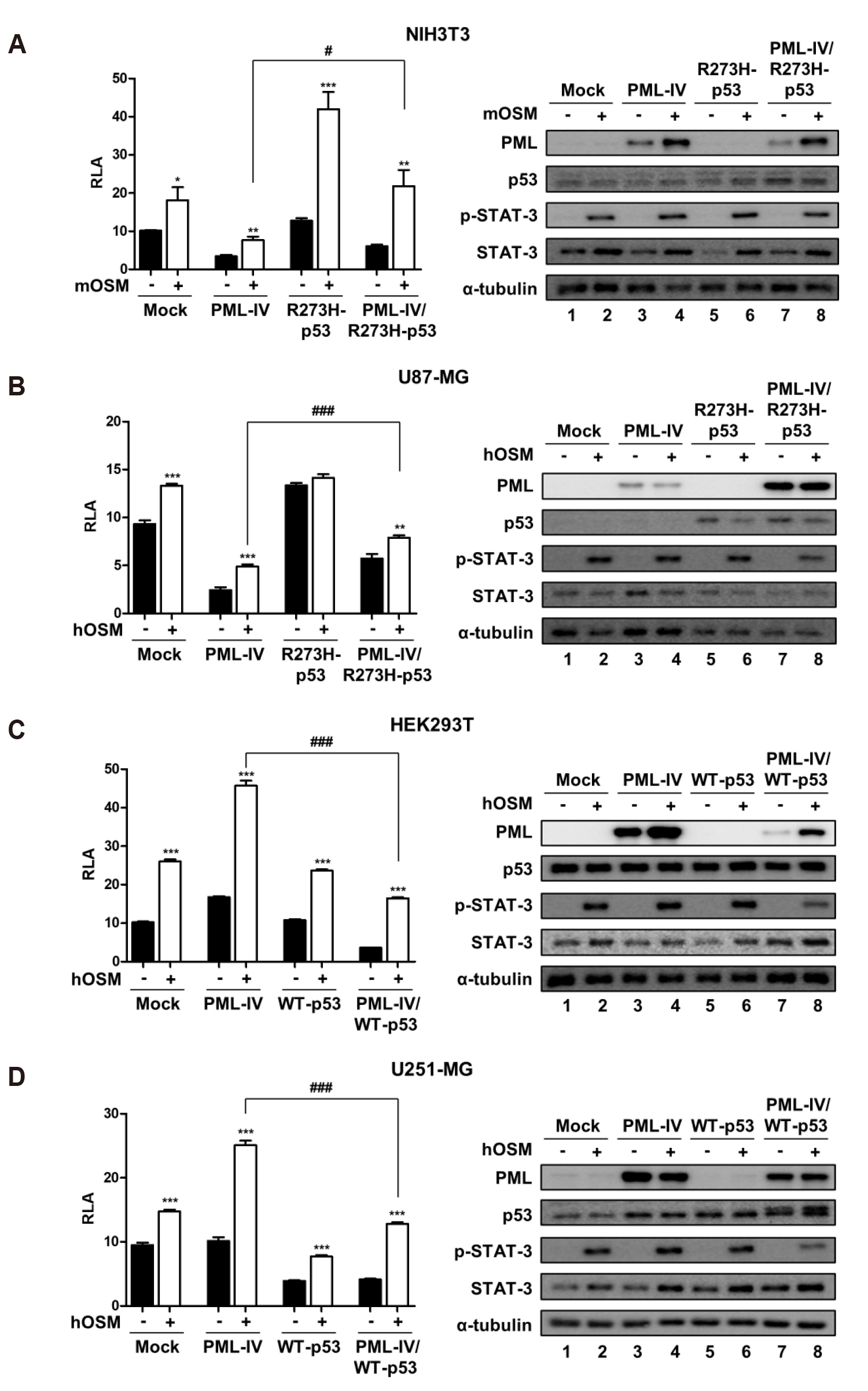
- Promyelocytic leukemia (PML) gene, through alternative splicing of its Cterminal region, generates several PML isoforms that interact with specific partners and perform distinct functions. The PML protein is a tumor suppressor that plays an important role by interacting with various proteins. Herein, we investigated the effect of the PML isoforms on oncostatin M (OSM)-induced signal transducer and activator of transcription-3 (STAT-3) transcriptional activity. PML influenced OSMinduced STAT-3 activity in a cell type-specific manner, which was dependent on the p53 status of the cells but regardless of PML isoform. Interestingly, overexpression of PML exerted opposite effects on OSM-induced STAT-3 activity in p53 wild-type and mutant cells. Specifically, overexpression of PML in the cell lines bearing wild-type p53 (NIH3T3 and U87-MG cells) decreased OSM-induced STAT-3 transcriptional activity, whereas overexpression of PML increased OSM-induced STAT-3 transcriptional activity in mutant p53-bearing cell lines (HEK293T and U251-MG cells). When wild-type p53 cells were co-transfected with PML-IV and R273H-p53 mutant, OSM-mediated STAT-3 transcriptional activity was significantly enhanced, compared to that of cells which were transfected with PML-IV alone; however, when cells bearing mutant p53 were co-transfected with PML-IV and wild-type p53, OSM-induced STAT-3 transcriptional activity was significantly decreased, compared to that of transfected cells with PML-IV alone. In conclusion, PML acts together with wild-type or mutant p53 and influences OSM-mediated STAT-3 activity in a negative or positive manner, resulting in the aberrant activation of STAT-3 in cancer cells bearing mutant p53 probably might occur through the interaction of mutant p53 with PML.
Keyword
Figure
Reference
-
1. Jensen K, Shiels C, Freemont PS. 2001; PML protein isoforms and the RBCC/TRIM motif. Oncogene. 20:7223–7233. DOI: 10.1038/sj.onc.1204765. PMID: 11704850.
Article2. Salomoni P, Ferguson BJ, Wyllie AH, Rich T. 2008; New insights into the role of PML in tumour suppression. Cell Res. 18:622–640. DOI: 10.1038/cr.2008.58. PMID: 18504460.
Article3. Nisole S, Maroui MA, Mascle XH, Aubry M, Chelbi-Alix MK. 2013; Differential roles of PML isoforms. Front Oncol. 3:125. DOI: 10.3389/fonc.2013.00125. PMID: 23734343. PMCID: PMC3660695.
Article4. Imani-Saber Z, Ghafouri-Fard S. 2014; Promyelocytic leukemia gene functions and roles in tumorigenesis. Asian Pac J Cancer Prev. 15:8021–8028. DOI: 10.7314/APJCP.2014.15.19.8019. PMID: 25338978.
Article5. Bernardi R, Pandolfi PP. 2007; Structure, dynamics and functions of promyelocytic leukaemia nuclear bodies. Nat Rev Mol Cell Biol. 8:1006–1016. DOI: 10.1038/nrm2277. PMID: 17928811.
Article6. Guan D, Kao HY. 2015; The function, regulation and therapeutic implications of the tumor suppressor protein, PML. Cell Biosci. 5:60. DOI: 10.1186/s13578-015-0051-9. PMID: 26539288. PMCID: PMC4632682.
Article7. Levy DE, Lee CK. 2002; What does Stat3 do? J Clin Invest. 109:1143–1148. DOI: 10.1172/JCI0215650. PMID: 11994402.
Article8. Huang S. 2007; Regulation of metastases by signal transducer and activator of transcription 3 signaling pathway: clinical implications. Clin Cancer Res. 13:1362–1366. DOI: 10.1158/1078-0432.CCR-06-2313. PMID: 17332277.9. Johnson DE, O'Keefe RA, Grandis JR. 2018; Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol. 15:234–248. DOI: 10.1038/nrclinonc.2018.8. PMID: 29405201. PMCID: PMC5858971.
Article10. Tanaka M, Miyajima A. 2003; Oncostatin M, a multifunctional cytokine. Rev Physiol Biochem Pharmacol. 149:39–52. DOI: 10.1007/s10254-003-0013-1. PMID: 12811586.11. Yu H, Lee H, Herrmann A, Buettner R, Jove R. 2014; Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nat Rev Cancer. 14:736–746. DOI: 10.1038/nrc3818. PMID: 25342631.
Article12. Laudisi F, Cherubini F, Monteleone G, Stolfi C. 2018; STAT3 interactors as potential therapeutic targets for cancer treatment. Int J Mol Sci. 19:E1787. DOI: 10.3390/ijms19061787. PMID: 29914167. PMCID: PMC6032216.
Article13. Junk DJ, Bryson BL, Jackson MW. 2014; HiJAK'd signaling; the STAT3 paradox in senescence and cancer progression. Cancers (Basel). 6:741–755. DOI: 10.3390/cancers6020741. PMID: 24675570. PMCID: PMC4074801.
Article14. West AJ, Tsui V, Stylli SS, Nguyen HPT, Morokoff AP, Kaye AH, Luwor RB. 2018; The role of interleukin-6-STAT3 signalling in glioblastoma. Oncol Lett. 16:4095–4104. DOI: 10.3892/ol.2018.9227. PMID: 30250528. PMCID: PMC6144698.15. Gottifredi V, Prives C. 2001; P53 and PML: new partners in tumor suppression. Trends Cell Biol. 11:184–187. DOI: 10.1016/S0962-8924(01)01983-3. PMID: 11316590.
Article16. Haupt S, di Agostino S, Mizrahi I, Alsheich-Bartok O, Voorhoeve M, Damalas A, Blandino G, Haupt Y. 2009; Promyelocytic leukemia protein is required for gain of function by mutant p53. Cancer Res. 69:4818–4826. DOI: 10.1158/0008-5472.CAN-08-4010. PMID: 19487292.
Article17. Yue X, Zhao Y, Xu Y, Zheng M, Feng Z, Hu W. 2017; Mutant p53 in cancer: accumulation, gain-of-function, and therapy. J Mol Biol. 429:1595–1606. DOI: 10.1016/j.jmb.2017.03.030. PMID: 28390900. PMCID: PMC5663274.
Article18. Guo A, Salomoni P, Luo J, Shih A, Zhong S, Gu W, Pandolfi PP. 2000; The function of PML in p53-dependent apoptosis. Nat Cell Biol. 2:730–736. DOI: 10.1038/35036365. PMID: 11025664.
Article19. Kawasaki A, Matsumura I, Kataoka Y, Takigawa E, Nakajima K, Kanakura Y. 2003; Opposing effects of PML and PML/RAR alpha on STAT3 activity. Blood. 101:3668–3673. DOI: 10.1182/blood-2002-08-2474. PMID: 12506013.20. Kato M, Muromoto R, Togi S, Iwakami M, Kitai Y, Kon S, Oritani K, Matsuda T. 2015; PML suppresses IL-6-induced STAT3 activation by interfering with STAT3 and HDAC3 interaction. Biochem Biophys Res Commun. 461:366–371. DOI: 10.1016/j.bbrc.2015.04.040. PMID: 25892518.
Article21. Choi YH, Bernardi R, Pandolfi PP, Benveniste EN. 2006; The promyelocytic leukemia protein functions as a negative regulator of IFN-gamma signaling. Proc Natl Acad Sci U S A. 103:18715–18720. DOI: 10.1073/pnas.0604800103. PMID: 17121994. PMCID: PMC1693728.22. Song C, Wang L, Wu X, Wang K, Xie D, Xiao Q, Li S, Jiang K, Liao L, Yates JR 3rd, Lee JD, Yang Q. 2018; PML Recruits TET2 to regulate DNA modification and cell proliferation in response to chemotherapeutic agent. Cancer Res. 78:2475–2489. DOI: 10.1158/0008-5472.CAN-17-3091. PMID: 29735542. PMCID: PMC6386530.
Article23. Liu Y, Wang JX, Huang D, Wang B, Li LL, Li XX, Ni P, Dong XL, Xia W, Yu CX, Xu WL, Chu WF, Zhao D. 2018; PMLIV overexpression promotes TGF-β-associated epithelial-mesenchymal transition and migration in MCF-7 cancer cells. J Cell Physiol. 233:9575–9583. DOI: 10.1002/jcp.26862. PMID: 29943817.24. Buschbeck M, Uribesalgo I, Ledl A, Gutierrez A, Minucci S, Muller S, Di Croce L. 2007; PML4 induces differentiation by Myc destabilization. Oncogene. 26:3415–3422. DOI: 10.1038/sj.onc.1210128. PMID: 17146439.
Article25. Oh W, Ghim J, Lee EW, Yang MR, Kim ET, Ahn JH, Song J. 2009; PML-IV functions as a negative regulator of telomerase by interacting with TERT. J Cell Sci. 122(Pt 15):2613–2622. DOI: 10.1242/jcs.048066. PMID: 19567472.
Article26. Kuo HY, Chen YC, Chang HY, Jeng JC, Lin EH, Pan CM, Chang YW, Wang ML, Chou YT, Shih HM, Wu CW. 2013; The PML isoform IV is a negative regulator of nuclear EGFR'stranscriptional activity in lung cancer. Carcinogenesis. 34:1708–1716. DOI: 10.1093/carcin/bgt109. PMID: 23563092.27. Ivanschitz L, Takahashi Y, Jollivet F, Ayrault O, Le Bras M, de Thé H. 2015; PML IV/ARF interaction enhances p53 SUMO-1 conjugation, activation, and senescence. Proc Natl Acad Sci U S A. 112:14278–14283. DOI: 10.1073/pnas.1507540112. PMID: 26578773. PMCID: PMC4655504.
Article28. Li C, Peng Q, Wan X, Sun H, Tang J. 2017; C-terminal motifs in promyelocytic leukemia protein isoforms critically regulate PML nuclear body formation. J Cell Sci. 130:3496–3506. DOI: 10.1242/jcs.202879. PMID: 28851805.
Article29. Lin J, Tang H, Jin X, Jia G, Hsieh JT. 2002; p53 regulates Stat3 phosphorylation and DNA binding activity in human prostate cancer cells expressing constitutively active Stat3. Oncogene. 21:3082–3088. DOI: 10.1038/sj.onc.1205426. PMID: 12082540.
Article30. Niu G, Wright KL, Ma Y, Wright GM, Huang M, Irby R, Briggs J, Karras J, Cress WD, Pardoll D, Jove R, Chen J, Yu H. 2005; Role of Stat3 in regulating p53 expression and function. Mol Cell Biol. 25:7432–7440. DOI: 10.1128/MCB.25.17.7432-7440.2005. PMID: 16107692. PMCID: PMC1190305.
Article31. Wörmann SM, Song L, Ai J, Diakopoulos KN, Kurkowski MU, Görgülü K, Ruess D, Campbell A, Doglioni C, Jodrell D, Neesse A, Demir IE, Karpathaki AP, Barenboim M, Hagemann T, Rose-John S, Sansom O, Schmid RM, Protti MP, Lesina M, Algül H. 2016; Loss of P53 function activates JAK2-STAT3 signaling to promote pancreatic tumor growth, stroma modification, and gemcitabine resistance in mice and is associated with patient survival. Gastroenterology. 151:180–193.e12. DOI: 10.1053/j.gastro.2016.03.010. PMID: 27003603.32. Schulz-Heddergott R, Stark N, Edmunds SJ, Li J, Conradi LC, Bohnenberger H, Ceteci F, Greten FR, Dobbelstein M, Moll UM. 2018; Therapeutic ablation of gain-of-function mutant p53 in colorectal cancer inhibits Stat3-mediated tumor growth and invasion. Cancer Cell. 34:298–314.e7. DOI: 10.1016/j.ccell.2018.07.004. PMID: 30107178. PMCID: PMC6582949.
Article33. de Stanchina E, Querido E, Narita M, Davuluri RV, Pandolfi PP, Ferbeyre G, Lowe SW. 2004; PML is a direct p53 target that modulates p53 effector functions. Mol Cell. 13:523–535. DOI: 10.1016/S1097-2765(04)00062-0. PMID: 14992722.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Oncostatin M on Invasion of Primary Trophoblasts under Normoxia and Hypoxia Conditions
- STAT3 and ERK Signaling Pathways Are Implicated in the Invasion Activity by Oncostatin M through Induction of Matrix Metalloproteinases 2 and 9
- An Association Study of the Signal Transducer and Activator of Transcription 6 Gene With Periodic Psychosis
- Two Cases of Leukemia Cutis Showing Clinical Improvement with Systemic Retinoid Administration
- Overview of Acute Promyelocytic Leukemia