J Korean Neurosurg Soc.
2020 Jan;63(1):34-44. 10.3340/jkns.2019.0067.
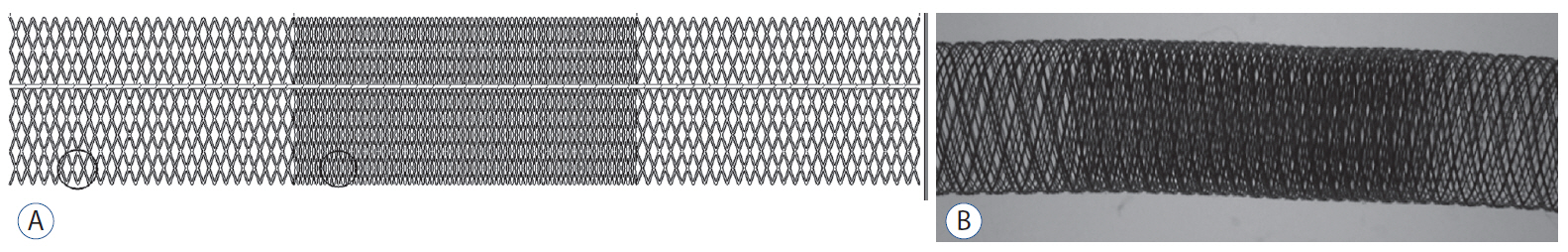
Healing of Aneurysm after Treatment Using Flow Diverter Stent : Histopathological Study in Experimental Canine Carotid Side Wall Aneurysm
- Affiliations
-
- 1Department of Neurosurgery, Hallym University Gangdong Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea
- 2Department of Radiology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- 3Department of Neurosurgery, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- 4Department of Neurosurgery, Veterans Health Service Medical Center, Seoul, Korea
- KMID: 2501691
- DOI: http://doi.org/10.3340/jkns.2019.0067
Abstract
Objective
: Despite widespread use of flow diverters (FDs) to treat aneurysms, the exact healing mechanism associated with FDs remains poorly understood. We aim to describe the healing process of aneurysms treated using FDs by demonstrating the histopathologic progression in a canine aneurysm model.
Methods
: Twenty-one side wall aneurysms were created in common carotid artery of eight dogs and treated with two different FDs. Angiographic follow-ups were done immediately after placement of the device, 4 weeks and 12 weeks. At last follow-up, the aneurysm and the device-implanted parent artery were harvested.
Results
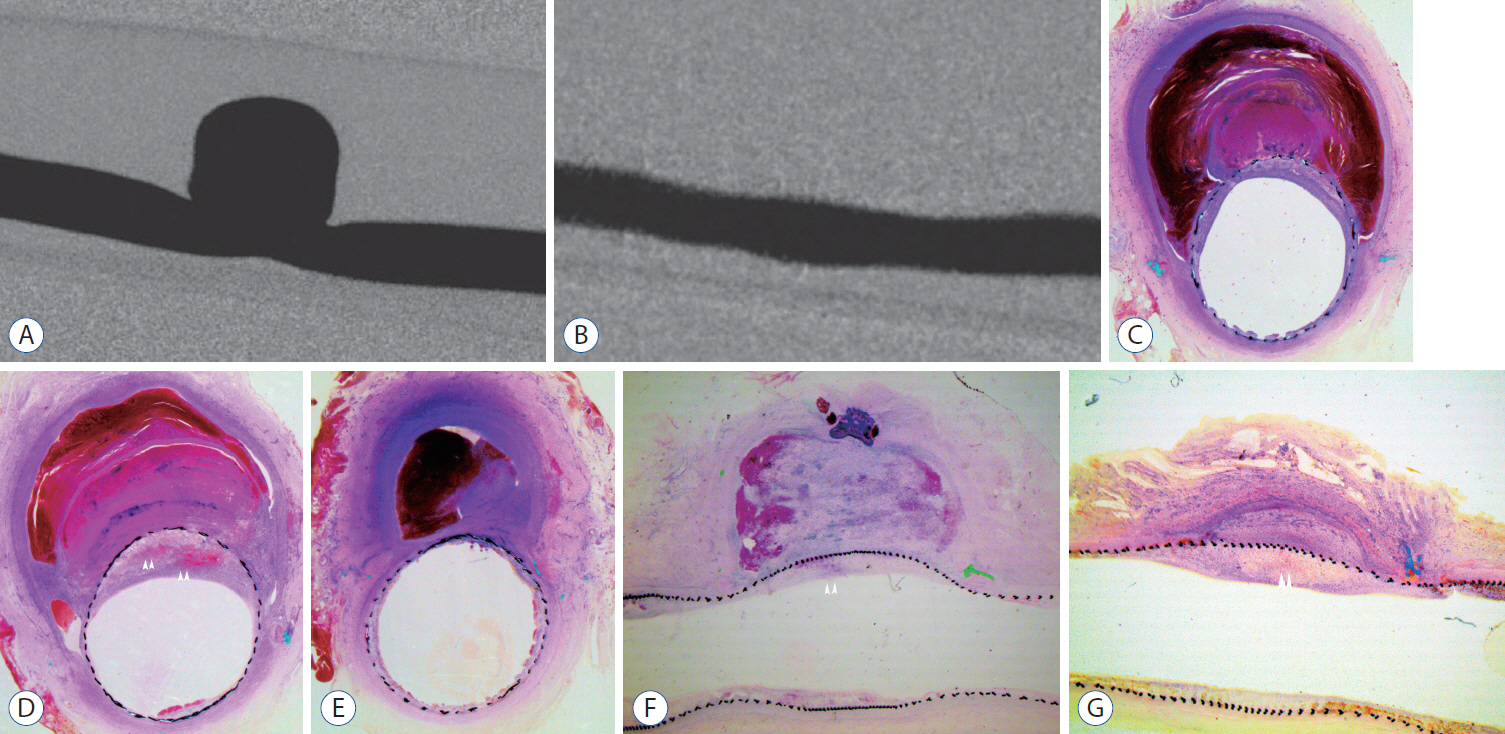
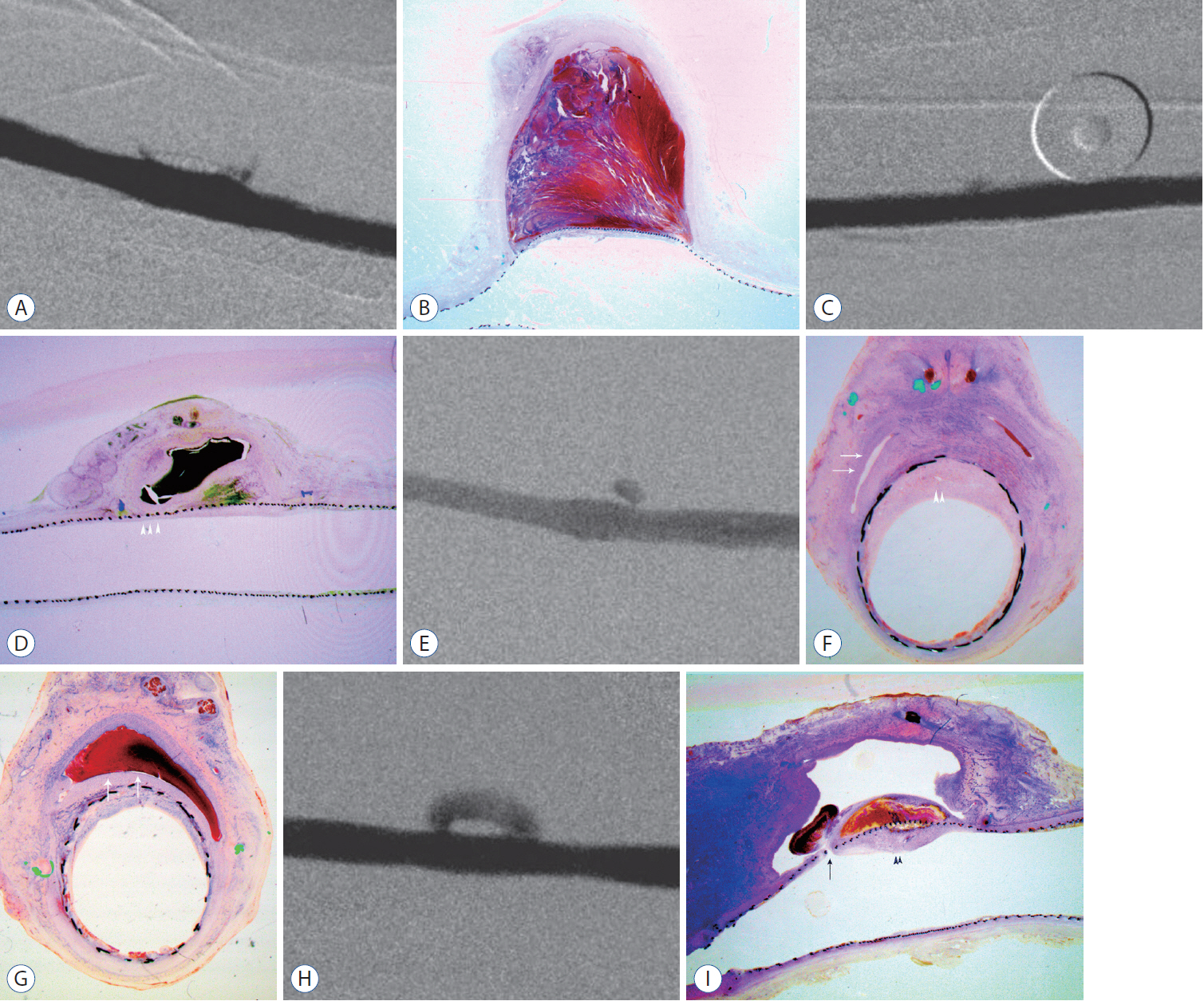
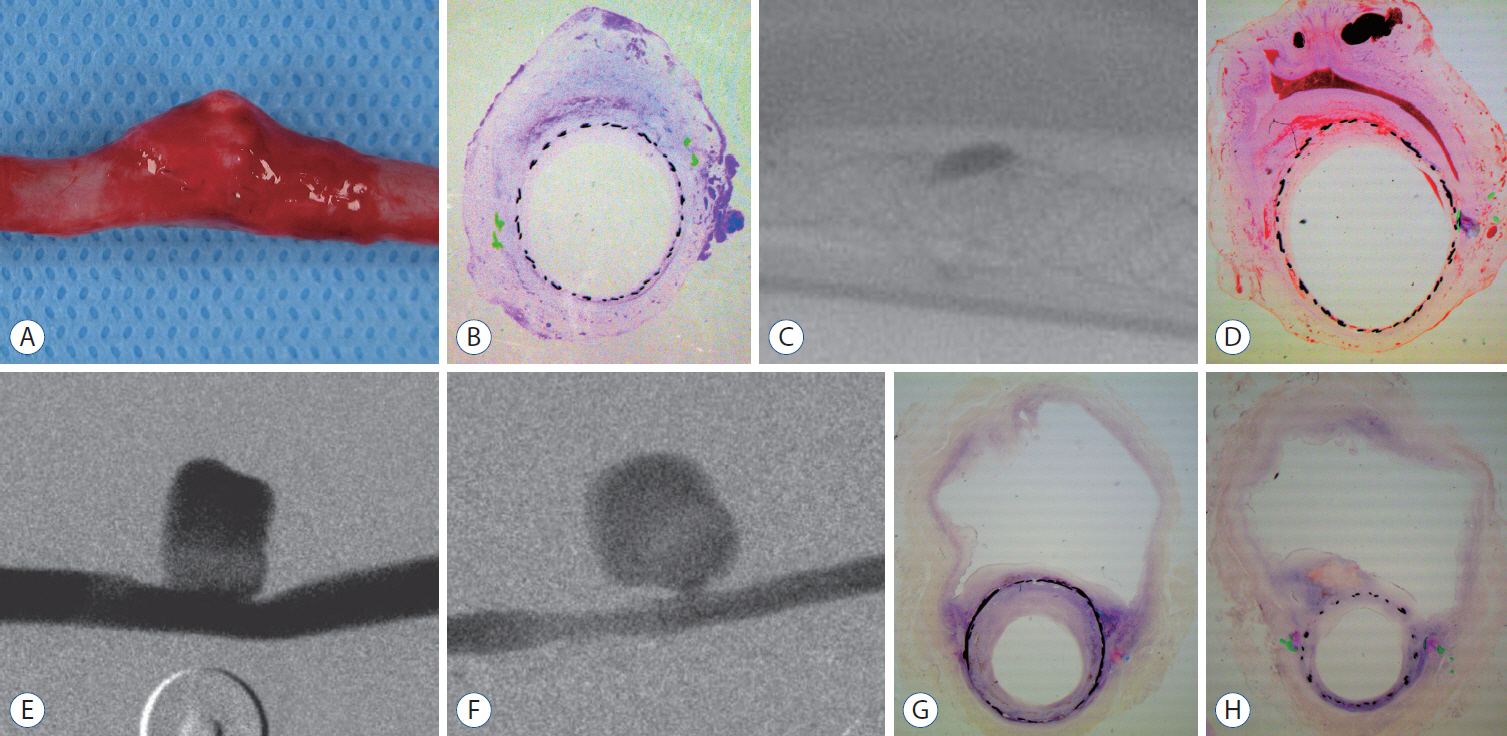
: Histopathologic findings of aneurysms at 4 weeks follow-up showed intra-aneurysm thrombus formation in laminating fashion, and neointimal thickening at the mid-segment of aneurysm. However, there are inhomogenous findings in aneurysms treated with the same type of FD showing same angiographic outcomes. At 12 weeks, aneurysms of complete and near-complete occlusion revealed markedly shrunken aneurysm filled with organized connective tissues with thin neointima. Aneurysms of incomplete occlusion at 12 weeks showed small amount of organized thrombus around fringe neck and large empty space with thick neointmal formation. Neointimal thickness and diameter stenosis was not significantly different between the groups of FD specification and follow-up period.
Conclusion
: Intra-aneurysmal thrombus formation and organization seem to be an important factor for the complete occlusion of aneurysms treated using the FD. Neointimal formation could occur along the struts of the FD independently of intra-aneurysmal thrombus formation. However, neointimal formation could not solely lead to complete aneurysm healing.
Figure
Cited by 1 articles
-
The Evolution of Flow-Diverting Stents for Cerebral Aneurysms; Historical Review, Modern Application, Complications, and Future Direction
Dong-Seong Shin, Christopher P. Carroll, Mohammed Elghareeb, Brian L. Hoh, Bum-Tae Kim
J Korean Neurosurg Soc. 2020;63(2):137-152. doi: 10.3340/jkns.2020.0034.
Reference
-
References
1. Arrese I, Sarabia R, Pintado R, Delgado-Rodriguez M. Flow-diverter devices for intracranial aneurysms: systematic review and meta-analysis. Neurosurgery. 73:193–199. discussion 199-200. 2013.2. Augsburger L, Farhat M, Reymond P, Fonck E, Kulcsar Z, Stergiopulos N, et al. Effect of flow diverter porosity on intraaneurysmal blood flow. Klin Neuroradiol. 19:204–214. 2009.
Article3. Baek JW, Huh CW, Heo YJ, Yoo MW, Kwon SC, Kwon OK, et al. Endovascular coiling of proximal middle cerebral artery aneurysms: is it safe and durable? Acta Neurochir (Wien). 160:2411–2418. 2018.
Article4. Baráth K, Cassot F, Fasel JH, Ohta M, Rufenacht DA. Influence of stent properties on the alteration of cerebral intra-aneurysmal haemodynamics: flow quantification in elastic sidewall aneurysm models. Neurol Res. 27 Suppl 1:S120–S128. 2005.
Article5. Bouzeghrane F, Naggara O, Kallmes DF, Berenstein A, Raymond J; International Consortium of Neuroendovascular Centres. In vivo experimental intracranial aneurysm models: a systematic review. AJNR Am J Neuroradiol. 31:418–423. 2010.
Article6. Briganti F, Napoli M, Tortora F, Solari D, Bergui M, Boccardi E, et al. Italian multicenter experience with flow-diverter devices for intracranial unruptured aneurysm treatment with periprocedural complications--a retrospective data analysis. Neuroradiology. 54:1145–1152. 2012.
Article7. Brinjikji W, Murad MH, Lanzino G, Cloft HJ, Kallmes DF. Endovascular treatment of intracranial aneurysms with flow diverters: a meta-analysis. Stroke. 44:442–447. 2013.
Article8. Cebral JR, Mut F, Raschi M, Hodis S, Ding YH, Erickson BJ, et al. Analysis of hemodynamics and aneurysm occlusion after flow-diverting treatment in rabbit models. AJNR Am J Neuroradiol. 35:1567–1573. 2014.
Article9. Chan TT, Chan KY, Pang PK, Kwok JC. Pipeline embolisation device for wide-necked internal carotid artery aneurysms in a hospital in Hong Kong: preliminary experience. Hong Kong Med J. 17:398–404. 2011.10. Cirillo L, Dall’olio M, Princiotta C, Simonetti L, Stafa A, Toni F, et al. The use of flow-diverting stents in the treatment of giant cerebral aneurysms: preliminary results. Neuroradiol J. 23:220–224. 2010.
Article11. Fernandez Zubillaga A, Guglielmi G, Viñuela F, Duckwiler GR. Endovascular occlusion of intracranial aneurysms with electrically detachable coils: correlation of aneurysm neck size and treatment results. AJNR Am J Neuroradiol. 15:815–820. 1994.12. Ionita CN, Natarajan SK, Wang W, Hopkins LN, Levy EI, Siddiqui AH, et al. Evaluation of a second-generation self-expanding variable-porosity flow diverter in a rabbit elastase aneurysm model. AJNR Am J Neuroradiol. 32:1399–1407. 2011.
Article13. Kadirvel R, Ding YH, Dai D, Rezek I, Lewis DA, Kallmes DF. Cellular mechanisms of aneurysm occlusion after treatment with a flow diverter. Radiology. 270:394–399. 2014.
Article14. Kallmes DF, Altes TA, Vincent DA, Cloft HJ, Do HM, Jensen ME. Experimental side-wall aneurysms: a natural history study. Neuroradiology. 41:338–341. 1999.
Article15. Kallmes DF, Ding YH, Dai D, Kadirvel R, Lewis DA, Cloft HJ. A second-generation, endoluminal, flow-disrupting device for treatment of saccular aneurysms. AJNR Am J Neuroradiol. 30:1153–1158. 2009.
Article16. Kallmes DF, Hanel R, Lopes D, Boccardi E, Bonafé A, Cekirge S, et al. International retrospective study of the pipeline embolization device: a multicenter aneurysm treatment study. AJNR Am J Neuroradiol. 36:108–115. 2015.
Article17. Kamran M, Yarnold J, Grunwald IQ, Byrne JV. Assessment of angiographic outcomes after flow diversion treatment of intracranial aneurysms: a new grading schema. Neuroradiology. 53:501–508. 2011.
Article18. Lee D, Yuki I, Murayama Y, Chiang A, Nishimura I, Vinters HV, et al. Thrombus organization and healing in the swine experimental aneurysm model. Part I. A histological and molecular analysis. J Neurosurg. 107:94–108. 2007.
Article19. Lee R, Adlam D, Clelland CA, Channon KM. Lines of Zahn in coronary artery thrombus. Eur Heart J. 33:1039. 2012.
Article20. Li Z, Zhao R, Fang X, Zhou J, Jiang G, Huang Q, et al. AMD3100 accelerates reendothelialization of neointima in rabbit saccular aneurysm after flow diverter treatment. World Neurosurg. 107:416–423. 2017.
Article21. Li ZF, Fang XG, Yang PF, Huang QH, Zhao WY, Liang C, et al. Endothelial progenitor cells contribute to neointima formation in rabbit elastase-induced aneurysm after flow diverter treatment. CNS Neurosci Ther. 19:352–357. 2013.
Article22. Massoud TF, Guglielmi G, Ji C, Viñuela F, Duckwiler GR. Experimental saccular aneurysms. I. Review of surgically-constructed models and their laboratory applications. Neuroradiology. 36:537–546. 1994.23. Meng H, Wang Z, Kim M, Ecker RD, Hopkins LN. Saccular aneurysms on straight and curved vessels are subject to different hemodynamics: implications of intravascular stenting. AJNR Am J Neuroradiol. 27:1861–1865. 2006.24. Oh SY, Kim MJ, Kim BS, Shin YS. Treatment for giant fusiform aneurysm located in the cavernous segment of the internal carotid artery using the pipeline embolization device. J Korean Neurosurg Soc. 55:32–35. 2014.
Article25. Sadasivan C, Cesar L, Seong J, Rakian A, Hao Q, Tio FO, et al. An original flow diversion device for the treatment of intracranial aneurysms: evaluation in the rabbit elastase-induced model. Stroke. 40:952–958. 2009.
Article26. Schafer AI. Genetic and acquired determinants of individual variability of response to antiplatelet drugs. Circulation. 108:910–911. 2003.
Article27. Szikora I, Berentei Z, Kulcsar Z, Marosfoi M, Vajda Z, Lee W, et al. Treatment of intracranial aneurysms by functional reconstruction of the parent artery: the Budapest experience with the Pipeline embolization device. AJNR Am J Neuroradiol. 31:1139–1147. 2010.
Article28. Turk AS, Aagaard-Kienitz B, Niemann D, Consigny D, Rappe A, Grinde J, et al. Natural history of the canine vein pouch aneurysm model. AJNR Am J Neuroradiol. 28:531–532. 2007.29. Yu SC, Kwok CK, Cheng PW, Chan KY, Lau SS, Lui WM, et al. Intracranial aneurysms: midterm outcome of pipeline embolization device--a prospective study in 143 patients with 178 aneurysms. Radiology. 265:893–901. 2012.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Delayed Proximal Flow Diverting Stent Migration in a Ruptured Intracranial Aneurysm: A Case Report
- Kinking of Flow Diverter in a Giant Wide-Necked Supraclinoid Internal Carotid Artery Aneurysm
- Symptomatic Post Endarterectomy Common Carotid Artery Pseudoaneurysm Treated with Combination of Flow Diverter Implantation and Carotid Stenting
- Salvage flow diverter stent across the posterior communicating artery for persistent retrograde filling of a giant internal carotid artery aneurysm after parent vessel occlusion
- Internal Carotid Artery Reconstruction with a “Mega Flow Diverterâ€: First Experience with the 6×50 mm DERIVO Embolization Device