Ann Rehabil Med.
2020 Feb;44(1):20-37. 10.5535/arm.2020.44.1.20.
Suggested Assessments for Sarcopenia in Patients With Stroke Who Can Walk Independently
- Affiliations
-
- 1Department of Rehabilitation Medicine, Konkuk University School of Medicine, Seoul, Korea
- 2International Healthcare Research Institute, Konkuk University, Seoul, Korea
- KMID: 2501043
- DOI: http://doi.org/10.5535/arm.2020.44.1.20
Abstract
Objective
To investigate variables for assessment of stroke-related sarcopenia that are alternative options to the current assessment for sarcopenia, which focuses on age-related sarcopenia and also has limitations in addressing sarcopenia due to weakness resulting from stroke.
Methods
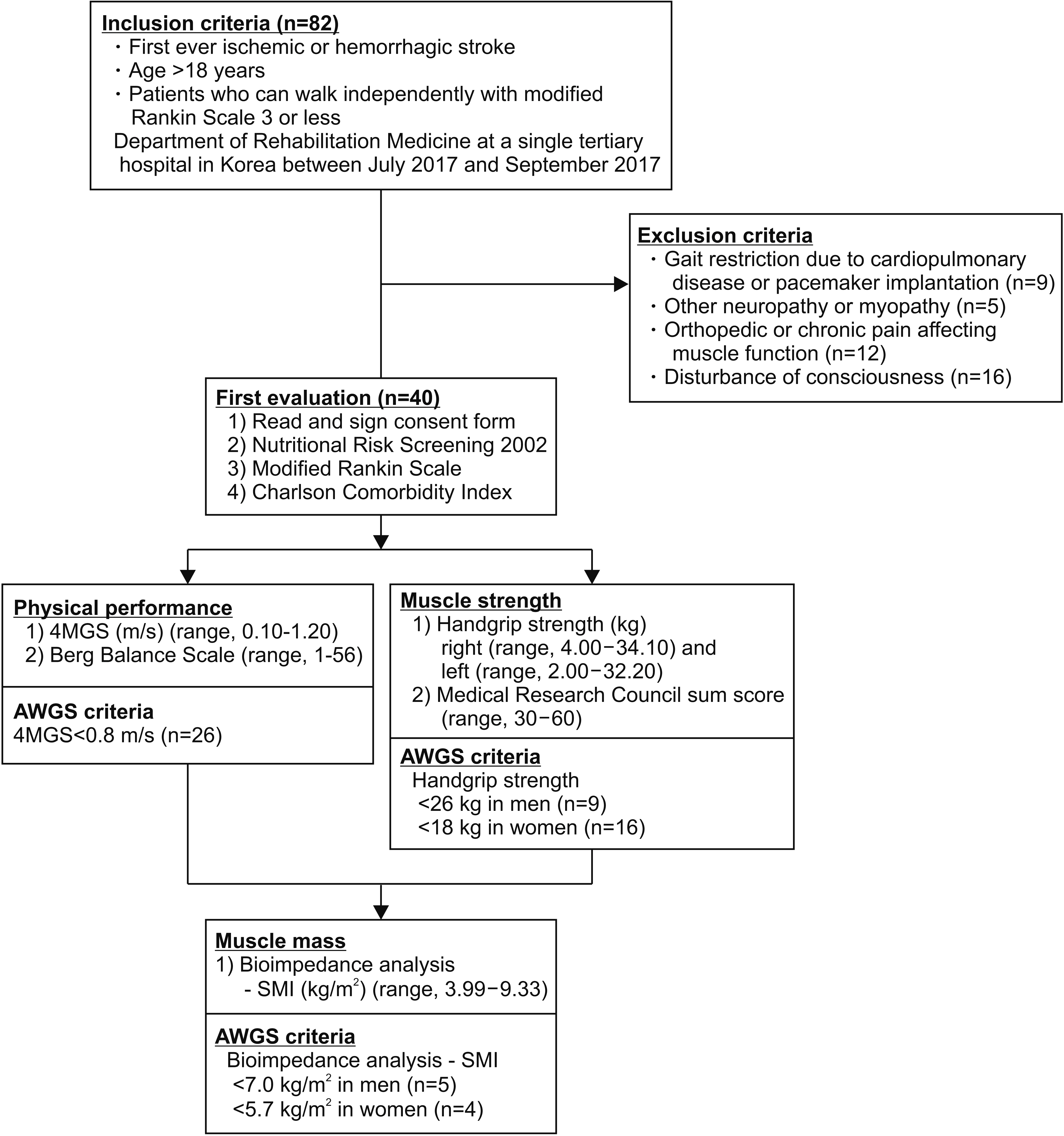
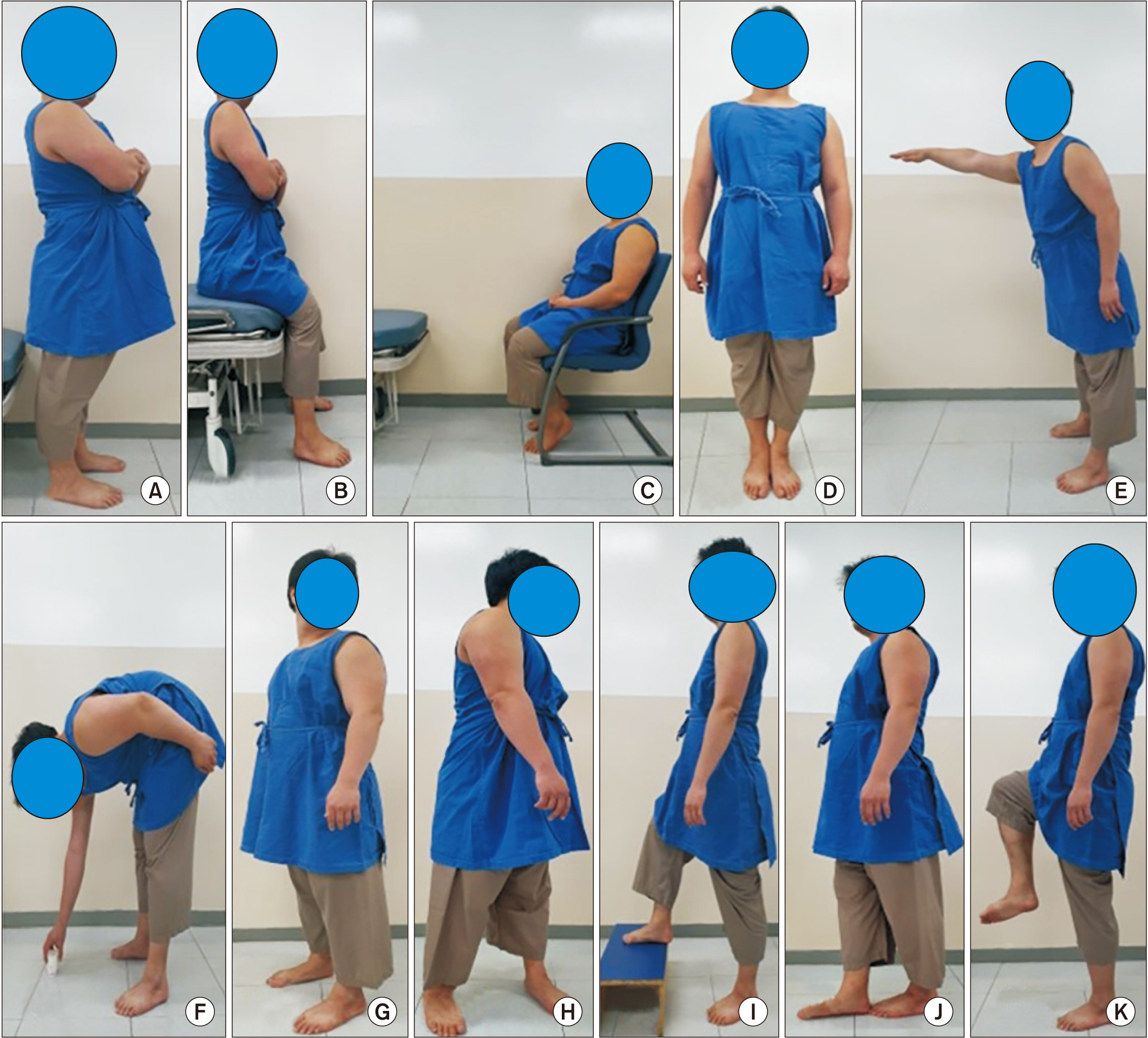
Forty patients (17 men, 23 women; mean age, 66.9±15.4 years) with first-ever stroke who can walk independently were included. Muscle mass was determined by measuring ultrasonographic muscle thickness of vastus intermedius, rectus femoris, tibialis anterior, medial gastrocnemius, and biceps brachii muscles in addition to using the skeletal muscle index (SMI) with bioelectrical impedance analysis. Muscle strength was assessed with the Medical Research Council (MRC) sum score as well as handgrip (HG) strength. Physical performance was measured by the Berg Balance Scale (BBS) along with 4-meter gait speed (4MGS). Correlations between each assessment in the three categories were analyzed and adjusted by stroke severity, comorbidity, and nutritional status.
Results
For muscle mass, SMI showed the highest correlation with the tibialis anterior muscle (r=0.783, p<0.001) among the other muscles. Regarding muscle strength, the MRC sum score correlated with the HG (r=0.660, p<0.001). For physical performance, the BBS correlated with the 4MGS (r=0.834, p<0.001). The same result was obtained after adjusting for factors of stroke severity, comorbidity, and nutritional status.
Conclusion
These results suggest that ultrasonographic muscle thickness of the tibialis anterior, the MRC sum score, and BBS might be alternatives to SMI, HG, and usual gait speed for sarcopenia in stroke patients.
Keyword
Figure
Cited by 1 articles
-
Association Between Length of Stay in the Intensive Care Unit and Sarcopenia Among Hemiplegic Stroke Patients
Aeri Jang, Chang Hoon Bae, Soo Jeong Han, Hasuk Bae
Ann Rehabil Med. 2021;45(1):49-56. doi: 10.5535/arm.20111.
Reference
-
1. Rosenberg IH. Summary comments. Am J Clin Nutr. 1989; 50:1231–3.
Article2. Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2014; 15:95–101.
Article3. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on sarcopenia in older people. Age Ageing. 2010; 39:412–23.
Article4. Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. J Am Med Dir Assoc. 2011; 12:249–56.5. Barillaro C, Liperoti R, Martone AM, Onder G, Landi F. The new metabolic treatments for sarcopenia. Aging Clin Exp Res. 2013; 25:119–27.
Article6. Rooks D, Praestgaard J, Hariry S, Laurent D, Petricoul O, Perry RG, et al. Treatment of sarcopenia with bimagrumab: results from a phase II, randomized, controlled, proof-of-concept study. J Am Geriatr Soc. 2017; 65:1988–95.
Article7. Aleman-Mateo H, Macias L, Esparza-Romero J, Astiazaran-Garcia H, Blancas AL. Physiological effects beyond the significant gain in muscle mass in sarcopenic elderly men: evidence from a randomized clinical trial using a protein-rich food. Clin Interv Aging. 2012; 7:225–34.
Article8. Scherbakov N, Sandek A, Doehner W. Stroke-related sarcopenia: specific characteristics. J Am Med Dir Assoc. 2015; 16:272–6.
Article9. World Health Organization. Global Health Estimates 2015: deaths by cause, age, sex, by country and by region, 2000-2015. Geneva, Switzerland: World Health Organization;2016.10. Kelly-Hayes M, Beiser A, Kase CS, Scaramucci A, D’Agostino RB, Wolf PA. The influence of gender and age on disability following ischemic stroke: the Framingham study. J Stroke Cerebrovasc Dis. 2003; 12:119–26.
Article11. Ticinesi A, Meschi T, Narici MV, Lauretani F, Maggio M. Muscle ultrasound and sarcopenia in older individuals: a clinical perspective. J Am Med Dir Assoc. 2017; 18:290–300.
Article12. Dalgas U, Severinsen K, Overgaard K. Relations between 6 minute walking distance and 10 meter walking speed in patients with multiple sclerosis and stroke. Arch Phys Med Rehabil. 2012; 93:1167–72.
Article13. Graham JE, Ostir GV, Fisher SR, Ottenbacher KJ. Assessing walking speed in clinical research: a systematic review. J Eval Clin Pract. 2008; 14:552–62.
Article14. Bohannon RW. Adequacy of hand-grip dynamometry for characterizing upper limb strength after stroke. Isokinet Exerc Sci. 2004; 12:263–5.
Article15. Ruiz JR, Sui X, Lobelo F, Morrow JR Jr, Jackson AW, Sjostrom M, et al. Association between muscular strength and mortality in men: prospective cohort study. BMJ. 2008; 337:a439.
Article16. Pillen S, van Alfen N. Skeletal muscle ultrasound. Neurol Res. 2011; 33:1016–24.
Article17. Gregson JM, Leathley MJ, Moore AP, Smith TL, Sharma AK, Watkins CL. Reliability of measurements of muscle tone and muscle power in stroke patients. Age Ageing. 2000; 29:223–8.18. Pradon D, Roche N, Enette L, Zory R. Relationship between lower limb muscle strength and 6-minute walk test performance in stroke patients. J Rehabil Med. 2013; 45:105–8.
Article19. Collin C, Wade D. Assessing motor impairment after stroke: a pilot reliability study. J Neurol Neurosurg Psychiatry. 1990; 53:576–9.
Article20. Lima CA, Ricci NA, Nogueira EC, Perracini MR. The Berg Balance Scale as a clinical screening tool to predict fall risk in older adults: a systematic review. Physiotherapy. 2018; 104:383–94.
Article21. Taghizadeh G, Martinez-Martin P, Fereshtehnejad SM, Habibi SA, Nikbakht N, Alizadeh NH, et al. Psychometric properties of the Berg balance scale in idiopathic Parkinson’ disease in the drug off-phase. Neurol Sci. 2018; 39:2175–81.
Article22. Blum L, Korner-Bitensky N. Usefulness of the Berg Balance Scale in stroke rehabilitation: a systematic review. Phys Ther. 2008; 88:559–66.
Article23. Wissel J, Schelosky LD, Scott J, Christe W, Faiss JH, Mueller J. Early development of spasticity following stroke: a prospective, observational trial. J Neurol. 2010; 257:1067–72.
Article24. Smithard DG, Smeeton NC, Wolfe CD. Long-term outcome after stroke: does dysphagia matter? Age Ageing. 2007; 36:90–4.
Article25. Moriwaki M, Wakabayashi H, Sakata K, Domen K. The effect of branched chain amino acids-enriched nutritional supplements on activities of daily living and muscle mass in inpatients with gait impairments: a randomized controlled trial. J Nutr Health Aging. 2019; 23:348–53.
Article26. Beaudart C, Reginster JY, Slomian J, Buckinx F, Dardenne N, Quabron A, et al. Estimation of sarcopenia prevalence using various assessment tools. Exp Gerontol. 2015; 61:31–7.
Article27. Arts IM, Pillen S, Schelhaas HJ, Overeem S, Zwarts MJ. Normal values for quantitative muscle ultrasonography in adults. Muscle Nerve. 2010; 41:32–41.
Article28. Fujiwara K, Asai H, Toyama H, Kunita K, Yaguchi C, Kiyota N, et al. Changes in muscle thickness of gastrocnemius and soleus associated with age and sex. Aging Clin Exp Res. 2010; 22:24–30.
Article29. Kon SS, Canavan JL, Nolan CM, Clark AL, Jones SE, Cullinan P, et al. The 4-metre gait speed in COPD: responsiveness and minimal clinically important difference. Eur Respir J. 2014; 43:1298–305.
Article30. Lee SH, Hwang BY. The correlations among the dynamic gait index the berg balance scale and timed up & go test in people with stroke. J Korean Phys Ther Sci. 2008; 15:1–8.31. van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988; 19:604–7.
Article32. D’Olhaberriague L, Litvan I, Mitsias P, Mansbach HH. A reappraisal of reliability and validity studies in stroke. Stroke. 1996; 27:2331–6.
Article33. Goldstein LB, Samsa GP, Matchar DB, Horner RD. Charlson Index comorbidity adjustment for ischemic stroke outcome studies. Stroke. 2004; 35:1941–5.
Article34. Kondrup J, Allison SP, Elia M, Vellas B, Plauth M; Educational and Clinical Practice Committee, et al. ESPEN guidelines for nutrition screening 2002. Clin Nutr. 2003; 22:415–21.
Article35. Kyle UG, Kossovsky MP, Karsegard VL, Pichard C. Comparison of tools for nutritional assessment and screening at hospital admission: a population study. Clin Nutr. 2006; 25:409–17.
Article36. Berenpas F, Martens AM, Weerdesteyn V, Geurts AC, van Alfen N. Bilateral changes in muscle architecture of physically active people with chronic stroke: a quantitative muscle ultrasound study. Clin Neurophysiol. 2017; 128:115–22.
Article37. Bertrand AM, Fournier K, Wick Brasey MG, Kaiser ML, Frischknecht R, Diserens K. Reliability of maximal grip strength measurements and grip strength recovery following a stroke. J Hand Ther. 2015; 28:356–62.
Article38. Bohannon RW. Is it legitimate to characterize muscle strength using a limited number of measures? J Strength Cond Res. 2008; 22:166–73.
Article39. Salbach NM, Mayo NE, Higgins J, Ahmed S, Finch LE, Richards CL. Responsiveness and predictability of gait speed and other disability measures in acute stroke. Arch Phys Med Rehabil. 2001; 82:1204–12.
Article40. Kobayashi T, Leung AK, Akazawa Y, Hutchins SW. Correlations between Berg balance scale and gait speed in individuals with stroke wearing ankle-foot orthoses: a pilot study. Disabil Rehabil Assist Technol. 2016; 11:219–22.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Rehabilitation Approaches in Stroke Patients with Underlying Sarcopenia: A Case Report and Literature Review
- Association of Sarcopenia and Its Components with Depression Symptoms in Older Patients with Stroke
- Low Hand Grip Strength, Mid-Upper Arm Muscle Area, Calf Circumference, Serum Albumin Level, and Muscle Fiber Diameter as Risk Factors for Independent Walking Inability in Patients with Hip Fracture 6 Weeks after Bipolar Hemiarthroplasty Surgery
- Diabetes and Sarcopenia
- Measurement of the Calf Muscle Circumference is Useful for Diagnosing Sarcopenia in Older Adults Requiring Long-Term Care