Ann Rehabil Med.
2020 Feb;44(1):1-10. 10.5535/arm.2020.44.1.1.
Epiglottic Retroflexion is a Key Indicator of Functional Recovery of Post-stroke Dysphagia
- Affiliations
-
- 1Department of Rehabilitation Medicine, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- 2National Traffic Injury Rehabilitation Hospital, Yangpyeong, Korea
- KMID: 2501041
- DOI: http://doi.org/10.5535/arm.2020.44.1.1
Abstract
Objective
To evaluate the longitudinal changes of swallowing kinematics based on videofluoroscopic swallowing studies (VFSSs) in subacute stroke patients grouped according to the method of dietary intake.
Methods
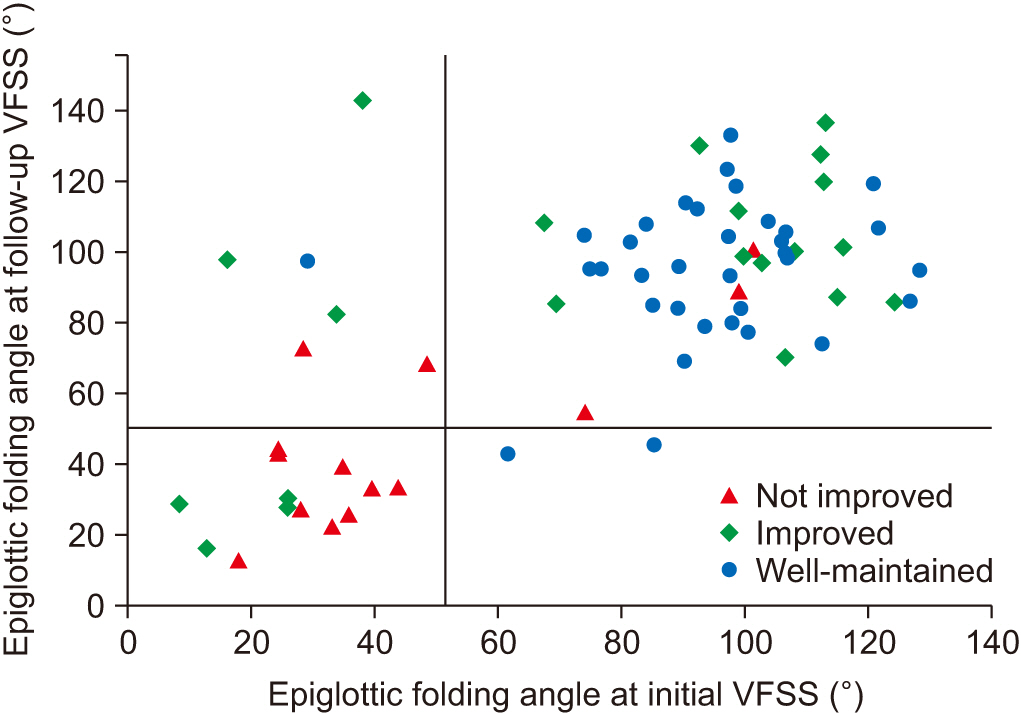
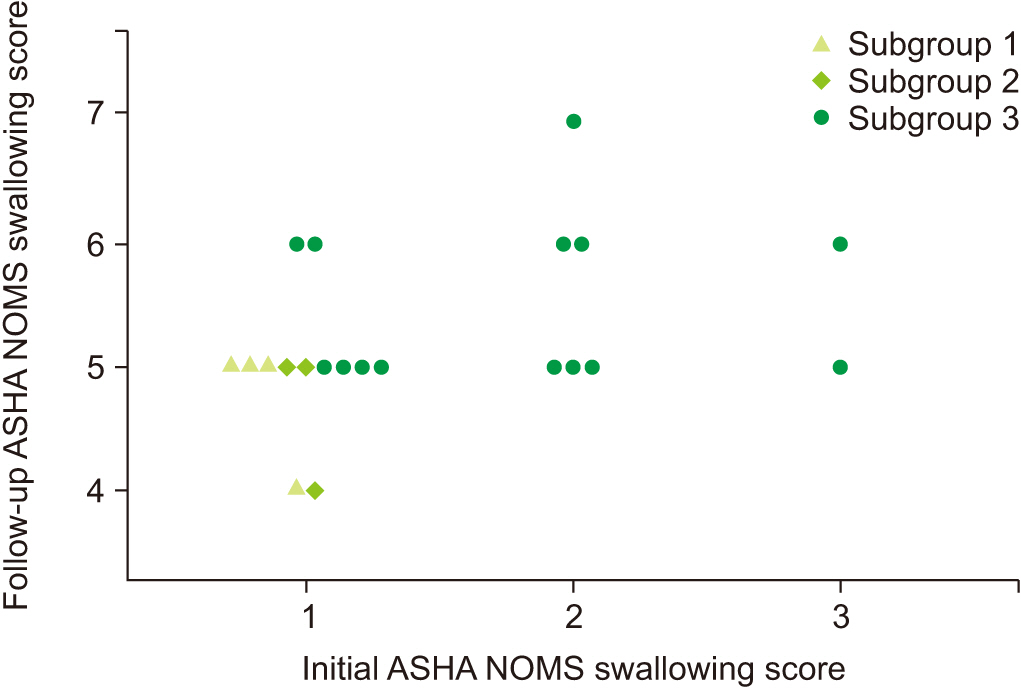
Sixty-nine subacute stroke patients who had taken at least 2 successive VFSSs were included. Subjects were allocated into 3 groups according to the degree of swallowing function recovery—not improved group (tube feeding recommended to patients at both studies), improved group (tube feedings recommended initially to patients and oral feeding recommended at follow-up study), and well-maintained group (oral feeding at both studies recommended to patients). Initial VFSS was performed during the subacute stage of stroke, 1 to 12 weeks after the onset of stroke, and follow-up VFSS was performed at least once. Kinematic variables were calculated by two-dimensional motion analysis of multiple structures, including the hyoid bone, epiglottis, and vocal cord. Changes of kinematic variables were analyzed in serial VFSSs.
Results
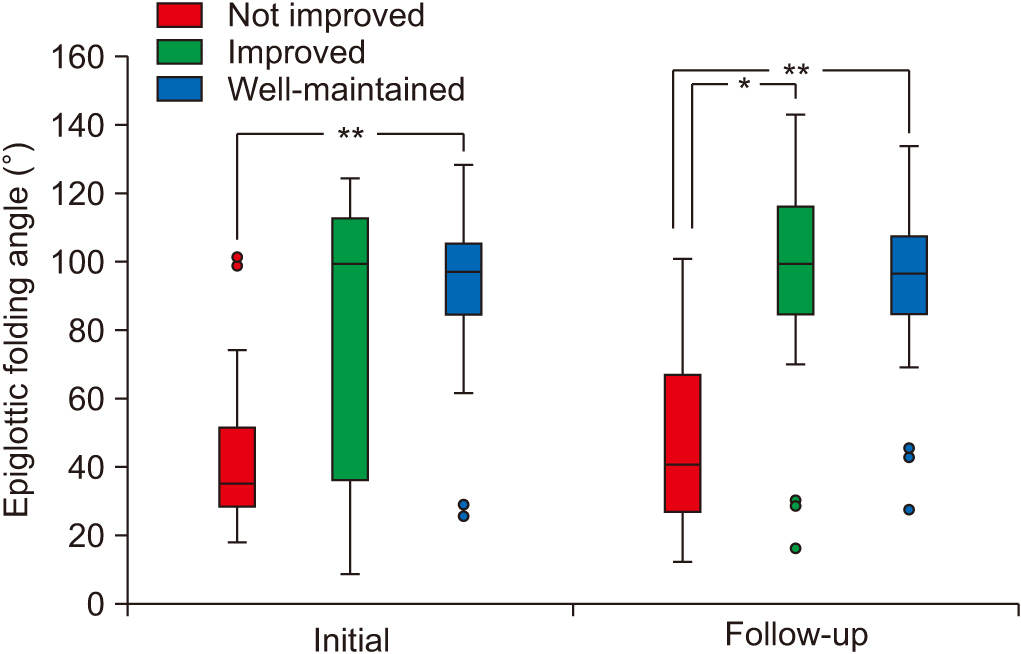
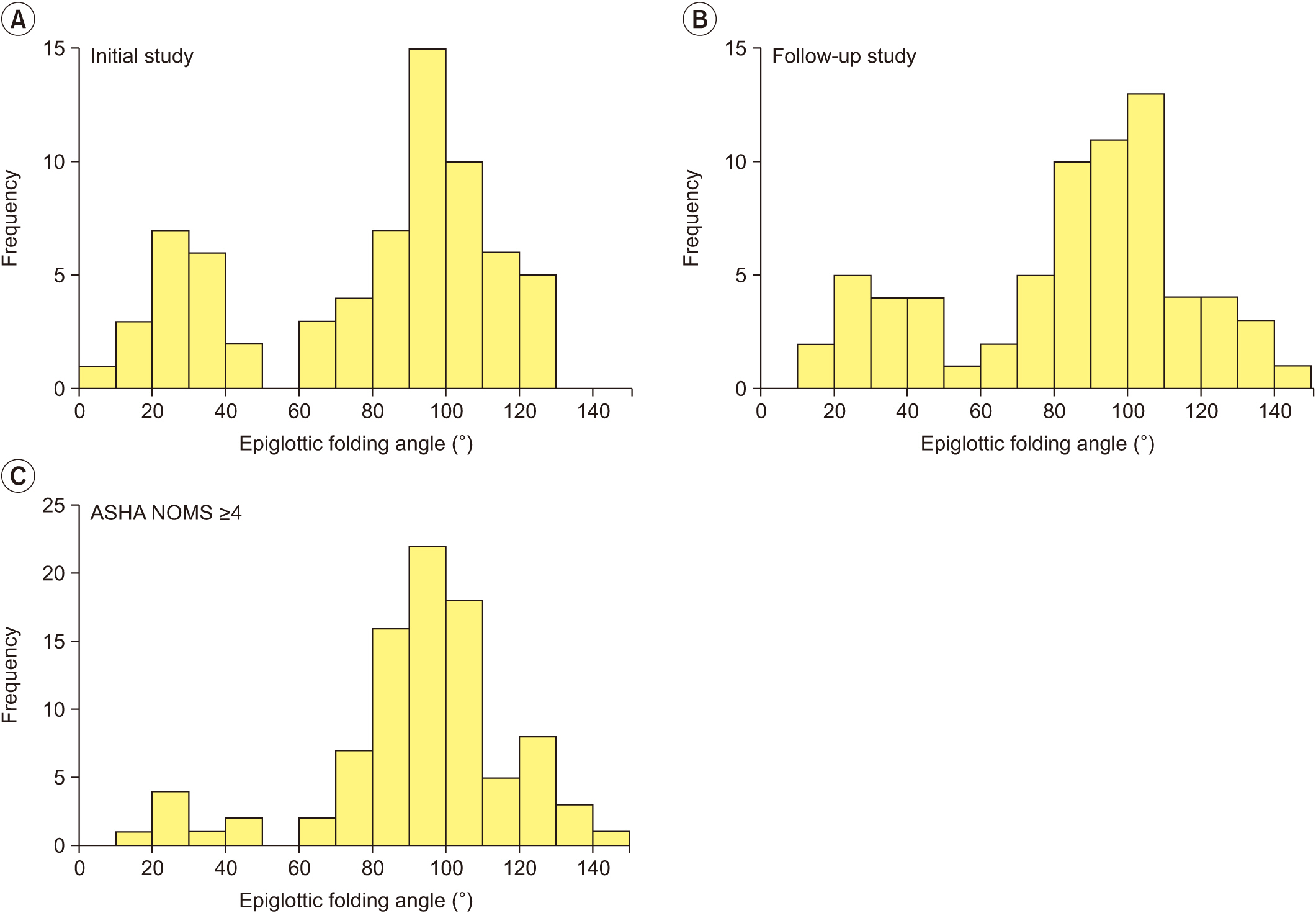
At the initial VFSS, the well-maintained group showed significantly larger angles of epiglottic folding than the not improved group, while at the follow-up VFSS, the improved and the well-maintained groups showed significantly larger epiglottic folding angles than the not improved group. The distribution of epiglottic folding angles was in a dichotomous pattern, and each cluster was related to the swallowing function.
Conclusion
This study showed that improved epiglottic folding angles are associated with the recovery of the swallowing process and suitability for oral feeding among various kinematic variables in subacute stroke patients.
Keyword
Figure
Cited by 1 articles
-
Correlation of Videofluoroscopic Swallowing Study Findings With Radionuclide Salivagram in Chronic Brain-Injured Patients
Ga Yang Shim, Ju Sun Oh, Seunghee Han, Kyungyeul Choi, Son Mi Lee, Min Woo Kim
Ann Rehabil Med. 2021;45(2):108-115. doi: 10.5535/arm.20171.
Reference
-
1. Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke. 2005; 36:2756–63.2. Foley NC, Martin RE, Salter KL, Teasell RW. A review of the relationship between dysphagia and malnutrition following stroke. J Rehabil Med. 2009; 41:707–13.
Article3. Daniels SK, Brailey K, Priestly DH, Herrington LR, Weisberg LA, Foundas AL. Aspiration in patients with acute stroke. Arch Phys Med Rehabil. 1998; 79:14–9.
Article4. Kim SB, Lee SJ, Lee KW, Lee JH, Kim DW. Usefulness of early videofluoroscopic swallowing study in acute stroke patients with dysphagia. Ann Rehabil Med. 2018; 42:42–51.
Article5. Holas MA, DePippo KL, Reding MJ. Aspiration and relative risk of medical complications following stroke. Arch Neurol. 1994; 51:1051–3.
Article6. Mar tin-Har r is B, L ogemann JA, McMahon S, Schleicher M, Sandidge J. Clinical utility of the modified barium swallow. Dysphagia. 2000; 15:136–41.
Article7. Hwang JM, Cheong YS, Kang MG, Chun SM, Min YS, Lee YS, et al. Recommendation of nasogastric tube removal in acute stroke patients based on videofluoroscopic swallow study. Ann Rehabil Med. 2017; 41:9–15.
Article8. Kim Y, McCullough GH, Asp CW. Temporal measurements of pharyngeal swallowing in normal populations. Dysphagia. 2005; 20:290–6.
Article9. Logemann JA, Pauloski BR, Rademaker AW, Colangelo LA, Kahrilas PJ, Smith CH. Temporal and biomechanical characteristics of oropharyngeal swallow in younger and older men. J Speech Lang Hear Res. 2000; 43:1264–74.
Article10. Paik NJ, Kim SJ, Lee HJ, Jeon JY, Lim JY, Han TR. Movement of the hyoid bone and the epiglottis during swallowing in patients with dysphagia from different etiologies. J Electromyogr Kinesiol. 2008; 18:329–35.
Article11. Kim Y, McCullough GH. Maximal hyoid excursion in poststroke patients. Dysphagia. 2010; 25:20–5.
Article12. Seo HG, Oh BM, Han TR. Longitudinal changes of the swallowing process in subacute stroke patients with aspiration. Dysphagia. 2011; 26:41–8.
Article13. Seo HG, Oh BM, Han TR. Swallowing kinematics and factors associated with laryngeal penetration and aspiration in stroke survivors with dysphagia. Dysphagia. 2016; 31:160–8.
Article14. Kwak HJ, Kim L, Ryu BJ, Kim YH, Park SW, Cho DG, et al. Influence of nasogastric tubes on swallowing in stroke patients: measuring hyoid bone movement with ultrasonography. Ann Rehabil Med. 2018; 42:551–9.
Article15. Wang ZY, Chen JM, Ni GX. Effect of an indwelling nasogastric tube on swallowing function in elderly poststroke dysphagia patients with long-term nasal feeding. BMC Neurol. 2019; 19:83.
Article16. Logemann JA. Manual for the videofluorographic study of swallowing. Austin, TX: Pro-ED;1993.17. Han TR, Paik NJ, Park JW, Kwon BS. The prediction of persistent dysphagia beyond six months after stroke. Dysphagia. 2008; 23:59–64.
Article18. Walfish S. A review of statistical outlier methods. Pharm Technol. 2006; 30:82–86.19. Choi KH, Ryu JS, Kim MY, Kang JY, Yoo SD. Kinematic analysis of dysphagia: significant parameters of aspiration related to bolus viscosity. Dysphagia. 2011; 26:392–8.
Article20. Nagy A, Molfenter SM, Peladeau-Pigeon M, Stokely S, Steele CM. The effect of bolus volume on hyoid kinematics in healthy swallowing. Biomed Res Int. 2014; 2014:738971.
Article21. Lenell C, Brates D, Pearson WG Jr, Molfenter S. Variations in healthy swallowing mechanics during various bolus conditions using computational analysis of swallowing mechanics (CASM). Dysphagia. 2019; Jun. 4. [Epub]. http://doi.org/10.1007/s00455-019-10026-9.
Article22. Logemann JA, Kahrilas PJ, Cheng J, Pauloski BR, Gibbons PJ, Rademaker AW, et al. Closure mechanisms of laryngeal vestibule during swallow. Am J Physiol. 1992; 262:G338–44.
Article23. Vandaele DJ, Perlman AL, Cassell MD. Intrinsic fibre architecture and attachments of the human epiglottis and their contributions to the mechanism of deglutition. J Anat. 1995; 186:1–15.24. Matsuo K, Palmer JB. Anatomy and physiology of feeding and swallowing: normal and abnormal. Phys Med Rehabil Clin N Am. 2008; 19:691–707.
Article25. Kim DH, Choi KH, Kim HM, Koo JH, Kim BR, Kim TW, et al. Inter-rater reliability of videofluoroscopic dysphagia scale. Ann Rehabil Med. 2012; 36:791–6.
Article26. Kim J, Oh BM, Kim JY, Lee GJ, Lee SA, Han TR. Validation of the videofluoroscopic dysphagia scale in various etiologies. Dysphagia. 2014; 29:438–43.
Article27. Henke C, Foerch C, Lapa S. Early screening parameters for dysphagia in acute ischemic stroke. Cerebrovasc Dis. 2017; 44:285–90.
Article28. Brown K, Cai C, Barreto A, Shoemaker P, Woellner J, Vu K, et al. Predictors of percutaneous endoscopic gastrostomy placement in acute ischemic stroke. J Stroke Cerebrovasc Dis. 2018; 27:3200–7.
Article29. Beharry A, Michel P, Faouzi M, Kuntzer T, Schweizer V, Diserens K. Predictive factors of swallowing disorders and bronchopneumonia in acute ischemic stroke. J Stroke Cerebrovasc Dis. 2019; 28:2148–54.
Article30. Kumar S, Doughty C, Doros G, Selim M, Lahoti S, Gokhale S, et al. Recovery of swallowing after dysphagic stroke: an analysis of prognostic factors. J Stroke Cerebrovasc Dis. 2014; 23:56–62.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Epidemiology, Natural Recovery, Long-term Outcome of Post Stroke Dysphagia
- Post-Stroke Dysphagia: Incidence, Complications and Pattern Relates to Brain Lesion
- Natural Course of Swallowing Recovery and Associated Factors in Post-Ischemic Stroke Dysphagia
- Interventional Management of Post-stroke Dysphagia
- Conservative Treatment of Dysphagia