Anesth Pain Med.
2020 Apr;15(2):133-142. 10.17085/apm.2020.15.2.133.
Perioperative management of patients receiving non-vitamin K antagonist oral anticoagulants: up-to-date recommendations
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Anesthesia and Pain Research Institute, Severance Cardiovascular Hospital, Yonsei University College of Medicine, Seoul, Korea
- KMID: 2500963
- DOI: http://doi.org/10.17085/apm.2020.15.2.133
Abstract
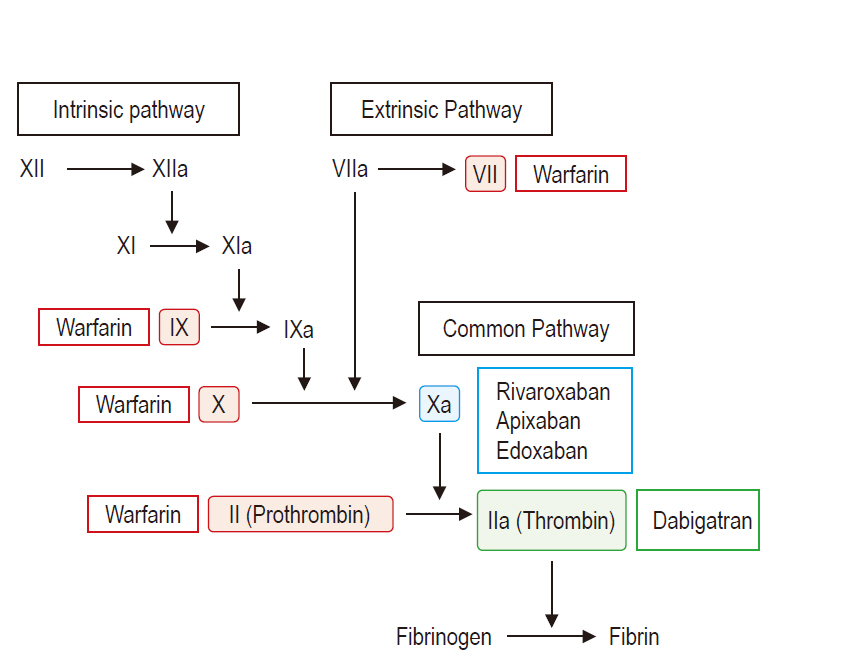
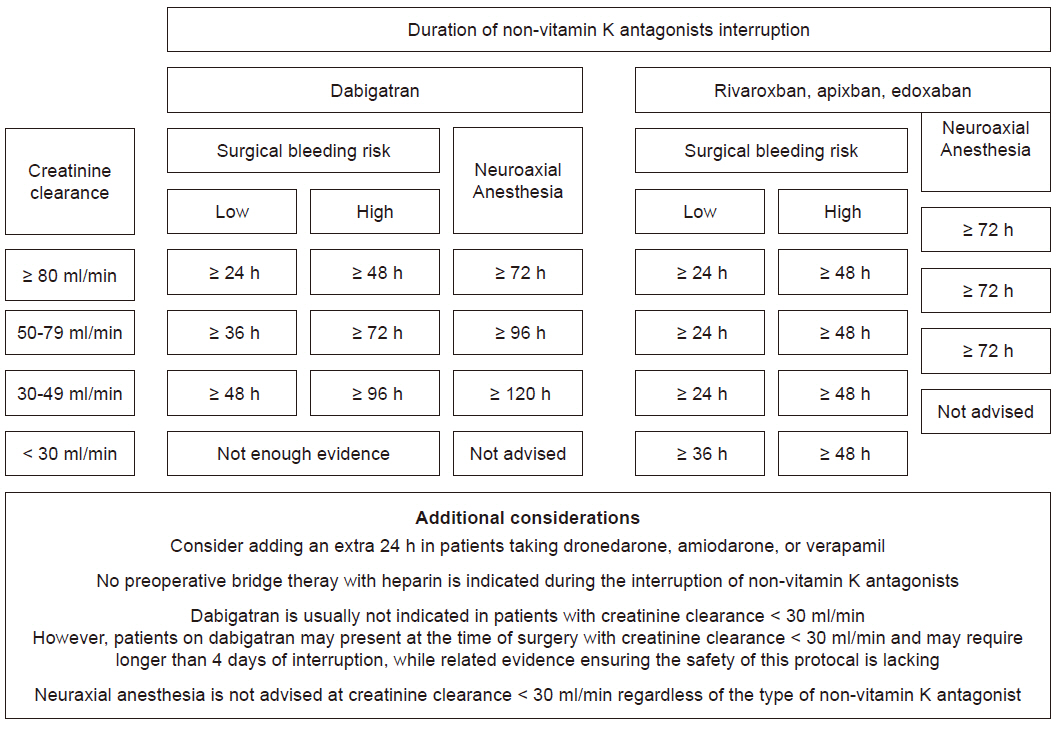
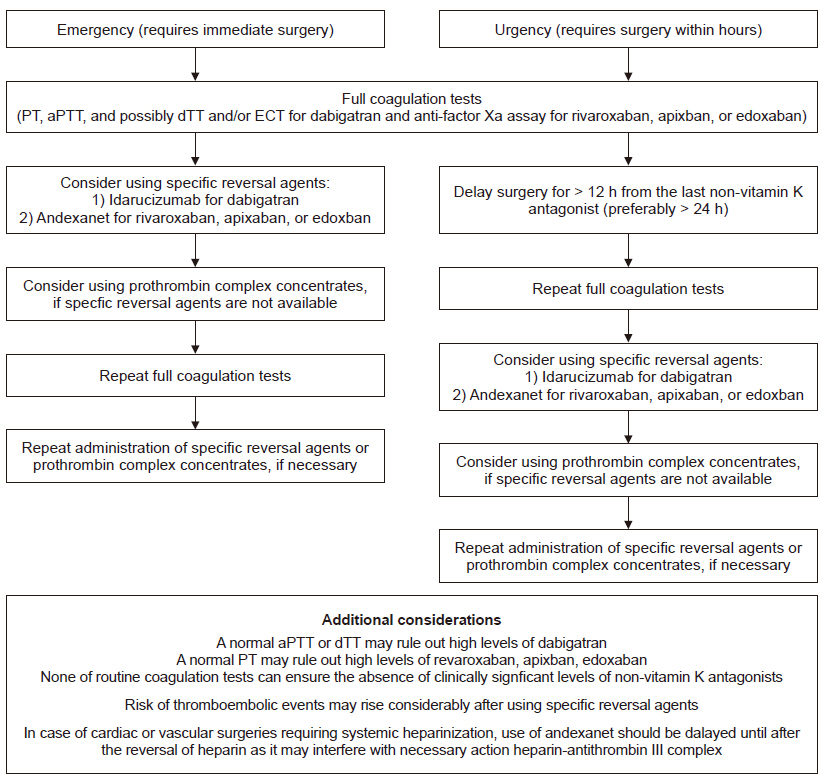
- Indications of non-vitamin K antagonist oral anticoagulants (NOACs), consisting of two types: direct thrombin inhibitor (dabigatran) and direct factor Xa inhibitor (rivaroxaban, apixaban, and edoxaban), have expanded over the last few years. Accordingly, increasing number of patients presenting for surgery are being exposed to NOACs, despite the fact that NOACs are inevitably related to increased perioperative bleeding risk. This review article contains recent clinical evidence-based up-to-date recommendations to help set up a multidisciplinary management strategy to provide a safe perioperative milieu for patients receiving NOACs. In brief, despite the paucity of related clinical evidence, several key recommendations can be drawn based on the emerging clinical evidence, expert consensus, and predictable pharmacological properties of NOACs. In elective surgeries, it seems safe to perform high-bleeding risk surgeries 2 days after cessation of NOAC, regardless of the type of NOAC. Neuraxial anesthesia should be performed 3 days after cessation of NOACs. In both instances, dabigatran needs to be discontinued for an additional 1 or 2 days, depending on the decrease in renal function. NOACs do not require a preoperative heparin bridge therapy. Emergent or urgent surgeries should preferably be delayed for at least 12 h from the last NOAC intake (better if > 24 h). If surgery cannot be delayed, consider using specific reversal agents, which are idarucizumab for dabigatran and andexanet alfa for rivaroxaban, apixaban, and edoxaban. If these specific reversal agents are not available, consider using prothrombin complex concentrates.
Figure
Reference
-
1. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, American College of Cardiology/American Heart Association Task Force on Practice Guidelines, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014; 64:e1–76.2. Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition). Chest. 2008; 133(6 Suppl):160S–98S.3. Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G. Oral anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012; 141(2 Suppl):e44S–88S.4. Yeh CH, Hogg K, Weitz JI. Overview of the new oral anticoagulants: opportunities and challenges. Arterioscler Thromb Vasc Biol. 2015; 35:1056–65.5. Levy JH, Faraoni D, Spring JL, Douketis JD, Samama CM. Managing new oral anticoagulants in the perioperative and intensive care unit setting. Anesthesiology. 2013; 118:1466–74.6. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, RE-LY Steering Committee and Investigators, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009; 361:1139–51.7. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, ROCKET AF Investigators, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011; 365:883–91.8. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, ARISTOTLE Committees and Investigators, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011; 365:981–92.9. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, ENGAGE AF-TIMI 48 Investigators, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013; 369:2093–104.10. Fanola CL, Giugliano RP, Ruff CT, Trevisan M, Nordio F, Mercuri MF, et al. A novel risk prediction score in atrial fibrillation for a net clinical outcome from the ENGAGE AF-TIMI 48 randomized clinical trial. Eur Heart J. 2017; 38:888–96.11. Ezekowitz MD, Nagarakanti R, Noack H, Brueckmann M, Litherland C, Jacobs M, et al. Comparison of dabigatran and warfarin in patients with atrial fibrillation and valvular heart disease: the RE-LY trial (randomized evaluation of long-term anticoagulant therapy). Circulation. 2016; 134:589–98.12. Piccini JP, Hellkamp AS, Washam JB, Becker RC, Breithardt G, Berkowitz SD, et al. Polypharmacy and the efficacy and safety of rivaroxaban versus warfarin in the prevention of stroke in patients with nonvalvular atrial fibrillation. Circulation. 2016; 133:352–60.13. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the Heart Rhythm Society in collaboration with the Society of Thoracic Surgeons. Circulation. 2019; 140:e125–51.14. Steffel J, Verhamme P, Potpara TS, Albaladejo P, Antz M, Desteghe L, et al. The 2018 European Heart Rhythm Association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. 2018; 39:1330–93.15. Chan NC, Eikelboom JW, Weitz JI. Evolving treatments for arterial and venous thrombosis: role of the direct oral anticoagulants. Circ Res. 2016; 118:1409–24.16. Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016; 149:315–52.17. Yeh CH, Gross PL, Weitz JI. Evolving use of new oral anticoagulants for treatment of venous thromboembolism. Blood. 2014; 124:1020–8.18. Eriksson BI, Quinlan DJ, Weitz JI. Comparative pharmacodynamics and pharmacokinetics of oral direct thrombin and factor xa inhibitors in development. Clin Pharmacokinet. 2009; 48:1–22.19. Levy JH, Spyropoulos AC, Samama CM, Douketis J. Direct oral anticoagulants: new drugs and new concepts. JACC Cardiovasc Interv. 2014; 7:1333–51.20. Raval AN, Cigarroa JE, Chung MK, Diaz-Sandoval LJ, Diercks D, Piccini JP, American Heart Association Clinical Pharmacology Subcommittee of the Acute Cardiac Care and General Cardiology Committee of the Council on Clinical Cardiology; Council on Cardiovascular Disease in the Young; and Council on Quality of Care and Outcomes Research, et al. Management of patients on non-vitamin K antagonist oral anticoagulants in the acute care and periprocedural setting: a scientific statement from the American Heart Association. Circulation. 2017; 135:e604–33.21. Godier A, Dincq AS, Martin AC, Radu A, Leblanc I, Antona M, et al. Predictors of pre-procedural concentrations of direct oral anticoagulants: a prospective multicentre study. Eur Heart J. 2017; 38:2431–9.22. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014; 383:955–62.23. Fang MC, Go AS, Chang Y, Hylek EM, Henault LE, Jensvold NG, et al. Death and disability from warfarin-associated intracranial and extracranial hemorrhages. Am J Med. 2007; 120:700–5.24. Beyer-Westendorf J, Gelbricht V, Förster K, Ebertz F, Köhler C, Werth S, et al. Peri-interventional management of novel oral anticoagulants in daily care: results from the prospective Dresden NOAC registry. Eur Heart J. 2014; 35:1888–96.25. Connolly SJ, Crowther M, Eikelboom JW, Gibson CM, Curnutte JT, Lawrence JH, ANNEXA-4 Investigators, et al. Full study report of andexanet alfa for bleeding associated with factor Xa inhibitors. N Engl J Med. 2019; 380:1326–35.26. Levy JH, Douketis J, Weitz JI. Reversal agents for non-vitamin K antagonist oral anticoagulants. Nat Rev Cardiol. 2018; 15:273–81.27. Pollack CV Jr, Reilly PA, van Ryn J, Eikelboom JW, Glund S, Bernstein RA, et al. Idarucizumab for dabigatran reversal - full cohort analysis. N Engl J Med. 2017; 377:431–41.28. Glund S, Moschetti V, Norris S, Stangier J, Schmohl M, van Ryn J, et al. A randomised study in healthy volunteers to investigate the safety, tolerability and pharmacokinetics of idarucizumab, a specific antidote to dabigatran. Thromb Haemost. 2015; 113:943–51.29. Glund S, Stangier J, Schmohl M, Gansser D, Norris S, van Ryn J, et al. Safety, tolerability, and efficacy of idarucizumab for the reversal of the anticoagulant effect of dabigatran in healthy male volunteers: a randomised, placebo-controlled, double-blind phase 1 trial. Lancet. 2015; 386:680–90.30. Crowther M, Crowther MA. Antidotes for novel oral anticoagulants: current status and future potential. Arterioscler Thromb Vasc Biol. 2015; 35:1736–45.31. Lu G, DeGuzman FR, Hollenbach SJ, Karbarz MJ, Abe K, Lee G, et al. A specific antidote for reversal of anticoagulation by direct and indirect inhibitors of coagulation factor Xa. Nat Med. 2013; 19:446–51.32. Sullivan DW Jr, Gad SC, Laulicht B, Bakhru S, Steiner S. Nonclinical safety assessment of PER977: a small molecule reversal agent for new oral anticoagulants and heparins. Int J Toxicol. 2015; 34:308–17.33. Ansell JE, Bakhru SH, Laulicht BE, Steiner SS, Grosso M, Brown K, et al. Use of PER977 to reverse the anticoagulant effect of edoxaban. N Engl J Med. 2014; 371:2141–2.34. Douketis JD, Berger PB, Dunn AS, Jaffer AK, Spyropoulos AC, Becker RC, et al. The perioperative management of antithrombotic therapy: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest. 2008; 133(6 Suppl):299S–339S.35. Healey JS, Eikelboom J, Douketis J, Wallentin L, Oldgren J, Yang S, et al. Periprocedural bleeding and thromboembolic events with dabigatran compared with warfarin: results from the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) randomized trial. Circulation. 2012; 126:343–8.36. Eikelboom JW, Kozek-Langenecker S, Exadaktylos A, Batorova A, Boda Z, Christory F, et al. Emergency care of patients receiving non-vitamin K antagonist oral anticoagulants. Br J Anaesth. 2018; 120:645–56.37. Kozek-Langenecker SA, Ahmed AB, Afshari A, Albaladejo P, Aldecoa C, Barauskas G, et al. Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology: first update 2016. Eur J Anaesthesiol. 2017; 34:332–95.38. Verma A, Ha ACT, Rutka JT, Verma S. What surgeons should know about non-vitamin K oral anticoagulants: a review. JAMA Surg. 2018; 153:577–85.39. Gallego P, Apostolakis S, Lip GY. Bridging evidence-based practice and practice-based evidence in periprocedural anticoagulation. Circulation. 2012; 126:1573–6.40. Douketis JD, Healey JS, Brueckmann M, Eikelboom JW, Ezekowitz MD, Fraessdorf M, et al. Perioperative bridging anticoagulation during dabigatran or warfarin interruption among patients who had an elective surgery or procedure. Substudy of the RE-LY trial. Thromb Haemost. 2015; 113:625–32.41. Sherwood MW, Douketis JD, Patel MR, Piccini JP, Hellkamp AS, Lokhnygina Y, ROCKET AF Investigators, et al. Outcomes of temporary interruption of rivaroxaban compared with warfarin in patients with nonvalvular atrial fibrillation: results from the rivaroxaban once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation (ROCKET AF). Circulation. 2014; 129:1850–9.42. Schulman S, Carrier M, Lee AY, Shivakumar S, Blostein M, Spencer FA, Periop Dabigatran Study Group, et al. Perioperative management of dabigatran: a prospective cohort study. Circulation. 2015; 132:167–73.43. Douketis JD, Spyropoulos AC, Duncan J, Carrier M, Le Gal G, Tafur AJ, et al. Perioperative management of patients with atrial fibrillation receiving a direct oral anticoagulant. JAMA Intern Med. 2019; 179:1469–78.44. Horlocker TT, Vandermeuelen E, Kopp SL, Gogarten W, Leffert LR, Benzon HT. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine evidence-based guidelines (fourth edition). Reg Anesth Pain Med. 2018; 43:263–309.45. Connolly SJ, Milling TJ Jr, Eikelboom JW, Gibson CM, Curnutte JT, Gold A, ANNEXA-4 Investigators, et al. Andexanet alfa for acute major bleeding associated with factor Xa inhibitors. N Engl J Med. 2016; 375:1131–41.46. Pollack CV Jr, Reilly PA, Eikelboom J, Glund S, Verhamme P, Bernstein RA, et al. Idarucizumab for dabigatran reversal. N Engl J Med. 2015; 373:511–20.47. Dickneite G, Hoffman M. Reversing the new oral anticoagulants with prothrombin complex concentrates (PCCs): what is the evidence? Thromb Haemost. 2014; 111:189–98.48. Grottke O, Aisenberg J, Bernstein R, Goldstein P, Huisman MV, Jamieson DG, et al. Efficacy of prothrombin complex concentrates for the emergency reversal of dabigatran-induced anticoagulation. Crit Care. 2016; 20:115.49. Raphael J, Mazer CD, Subramani S, Schroeder A, Abdalla M, Ferreira R, et al. Society of Cardiovascular Anesthesiologists clinical practice improvement advisory for management of perioperative bleeding and hemostasis in cardiac surgery patients. Anesth Analg. 2019; 129:1209–21.50. Kaatz S, Crowther M. Reversal of target-specific oral anticoagulants. J Thromb Thrombolysis. 2013; 36:195–202.51. Eikelboom JW, Quinlan DJ, Hirsh J, Connolly SJ, Weitz JI. Laboratory monitoring of non-vitamin K antagonist oral anticoagulant use in patients with atrial fibrillation: a review. JAMA Cardiol. 2017; 2:566–74.52. Cuker A. Laboratory measurement of the non-vitamin K antagonist oral anticoagulants: selecting the optimal assay based on drug, assay availability, and clinical indication. J Thromb Thrombolysis. 2016; 41:241–7.53. Dale BJ, Chan NC, Eikelboom JW. Laboratory measurement of the direct oral anticoagulants. Br J Haematol. 2016; 172:315–36.54. van Ryn J, Grottke O, Spronk H. Measurement of dabigatran in standardly used clinical assays, whole blood viscoelastic coagulation, and thrombin generation assays. Clin Lab Med. 2014; 34:479–501.55. Khadzhynov D, Wagner F, Formella S, Wiegert E, Moschetti V, Slowinski T, et al. Effective elimination of dabigatran by haemodialysis. A phase I single-centre study in patients with end-stage renal disease. Thromb Haemost. 2013; 109:596–605.56. Siegal DM, Garcia DA, Crowther MA. How I treat target-specific oral anticoagulant-associated bleeding. Blood. 2014; 123:1152–8.57. Heidbuchel H, Verhamme P, Alings M, Antz M, Diener HC, Hacke W, et al. Updated European Heart Rhythm Association practical guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation. Europace. 2015; 17:1467–507.58. Bromley A, Plitt A. A review of the role of non-vitamin K oral anticoagulants in the acute and long-term treatment of venous thromboembolism. Cardiol Ther. 2018; 7:1–13.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Underutilization of anticoagulants in patients with nonvalvular atrial fibrillation in the era of non‑vitamin K antagonist oral anticoagulants
- Adaptation of New Oral Anticoagulants for Warfarin Anticoagulated Patient with Traumatic Ongoing Hemorrhage
- The 2018 Korean Heart Rhythm Society Practical Guidelines on the use of Non-Vitamin K-Antagonist Oral Anticoagulants: Bleeding Control and Perioperative Management
- Periprocedural antithrombotic management
- 2018 Korean Heart Rhythm Society Guidelines for Non-Vitamin K Antagonist Oral Anticoagulants