Ann Surg Treat Res.
2020 Apr;98(4):184-189. 10.4174/astr.2020.98.4.184.
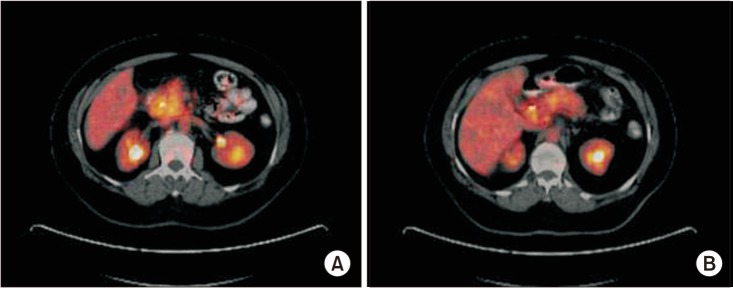
The efficacy of 18F-FDG PET/CT in the preoperative evaluation of pancreatic lesions
- Affiliations
-
- 1Department of General Surgery, Faculty of Medicine, Cukurova University, Adana, Turkey
- 2Department of Surgical Oncology, Faculty of Medicine, Cukurova University, Adana, Turkey
- 3Department of Nuclear Medicine, Faculty of Medicine, Cukurova University, Adana, Turkey
- KMID: 2500818
- DOI: http://doi.org/10.4174/astr.2020.98.4.184
Abstract
- Purpose
Since the treatment strategy for benign and malignant pancreatic lesions differ, we aimed to evaluate the clinical value of PET/CT in the diagnosis and management of pancreatic lesions.
Methods
Ninety patients who had a histologically confirmed pancreatic lesion were studied. Receiver operating characteristic (ROC) curve analysis was used to investigate the ability of PET/CT to differentiate malignant lesions from benign tumors.
Results
The malignant and benign groups comprised 64 and 26 patients, respectively. Despite the similarity in the size of primary tumors (P = 0.588), the mean maximum standardized uptake values (SUVmax) obtained from PET/CT imaging were significantly higher in malignant lesions (9.36 ± 5.9) than those of benign tumors (1.04 ± 2.6, P < 0.001). ROC analysis showed that the optimal SUVmax cutoff value for differentiating malignant lesions (to an accuracy of 91%; 95% confidence interval, 83%–98%) from benign tumors was 3.9 (sensitivity, 92.2%; specificity, 84.6%).
Conclusion
PET/CT evaluation of pancreatic lesions confers advantages including fine assessment of malignant potential with high sensitivity and accuracy using a threshold SUVmax value of 3.9.
Keyword
Figure
Reference
-
1. Sener SF, Fremgen A, Menck HR, Winchester DP. Pancreatic cancer: a report of treatment and survival trends for 100,313 patients diagnosed from 1985-1995, using the National Cancer Database. J Am Coll Surg. 1999; 189:1–7. PMID: 10401733.2. Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004; 363:1049–1057. PMID: 15051286.
Article3. Varadhachary GR, Tamm EP, Abbruzzese JL, Xiong HQ, Crane CH, Wang H, et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol. 2006; 13:1035–1046. PMID: 16865597.
Article4. Kauhanen SP, Komar G, Seppanen MP, Dean KI, Minn HR, Kajander SA, et al. A prospective diagnostic accuracy study of 18F-fluorodeoxyglucose positron emission tomography/computed tomography, multidetector row computed tomography, and magnetic resonance imaging in primary diagnosis and staging of pancreatic cancer. Ann Surg. 2009; 250:957–963. PMID: 19687736.
Article5. Okamoto K, Koyama I, Miyazawa M, Toshimitsu Y, Aikawa M, Okada K, et al. Preoperative 18[F]-fluorodeoxyglucose positron emission tomography/computed tomography predicts early recurrence after pancreatic cancer resection. Int J Clin Oncol. 2011; 16:39–44. PMID: 20862596.
Article6. Nakamoto Y, Higashi T, Sakahara H, Tamaki N, Kogire M, Doi R, et al. Delayed (18)F-fluoro-2-deoxy-D-glucose positron emission tomography scan for differentiation between malignant and benign lesions in the pancreas. Cancer. 2000; 89:2547–2554. PMID: 11135214.7. Diederichs CG, Staib L, Vogel J, Glasbrenner B, Glatting G, Brambs HJ, et al. Values and limitations of 18F-fluorodeoxyglucose-positron-emission tomography with preoperative evaluation of patients with pancreatic masses. Pancreas. 2000; 20:109–116. PMID: 10707924.
Article8. Delbeke D, Rose DM, Chapman WC, Pinson CW, Wright JK, Beauchamp RD, et al. Optimal interpretation of FDG PET in the diagnosis, staging and management of pancreatic carcinoma. J Nucl Med. 1999; 40:1784–1791. PMID: 10565771.9. Hosten N, Lemke AJ, Wiedenmann B, Bohmig M, Rosewicz S. Combined imaging techniques for pancreatic cancer. Lancet. 2000; 356:909–910. PMID: 11036898.
Article10. Beyer T, Townsend DW, Brun T, Kinahan PE, Charron M, Roddy R, et al. A combined PET/CT scanner for clinical oncology. J Nucl Med. 2000; 41:1369–1379. PMID: 10945530.11. Busing KA, Schonberg SO, Brade J, Wasser K. Impact of blood glucose, diabetes, insulin, and obesity on standardized uptake values in tumors and healthy organs on 18F-FDG PET/CT. Nucl Med Biol. 2013; 40:206–213. PMID: 23228852.12. Kim HS, Jang JY, Han Y, Lee KB, Joo I, Lee DH, et al. Survival outcome and prognostic factors of neoadjuvant treatment followed by resection for borderline resectable pancreatic cancer. Ann Surg Treat Res. 2017; 93:186–194. PMID: 29094028.
Article13. Higashi T, Saga T, Nakamoto Y, Ishimori T, Mamede MH, Wada M, et al. Relationship between retention index in dual-phase (18)F-FDG PET, and hexokinase-II and glucose transporter-1 expression in pancreatic cancer. J Nucl Med. 2002; 43:173–180. PMID: 11850481.14. Kitasato Y, Yasunaga M, Okuda K, Kinoshita H, Tanaka H, Okabe Y, et al. Maximum standardized uptake value on 18F-fluoro-2-deoxy-glucose positron emi ssion tomog raphy/computed tomography and glucose transporter-1 expression correlates with survival in invasive ductal carcinoma of the pancreas. Pancreas. 2014; 43:1060–1065. PMID: 25121413.15. Mansour JC, Schwartz L, Pandit-Taskar N, D'Angelica M, Fong Y, Larson SM, et al. The utility of F-18 fluorodeoxyglucose whole body PET imaging for determining malignancy in cystic lesions of the pancreas. J Gastrointest Surg. 2006; 10:1354–1360. PMID: 17175454.
Article16. Kauhanen S, Rinta-Kiikka I, Kemppainen J, Gronroos J, Kajander S, Seppanen M, et al. Accuracy of 18F-FDG PET/CT, multidetector CT, and MR imaging in the diagnosis of pancreatic cysts: a prospective single-center study. J Nucl Med. 2015; 56:1163–1168. PMID: 26045314.
Article17. Sato M, Hiyama T, Kato K, Shirasu T, Yoshimi F, Nagai H, et al. F-18 FDG accumulation in mucinous cystic neoplasm of pancreas. Clin Nucl Med. 2011; 36:45–48. PMID: 21157210.
Article18. Shreve PD. Focal fluorine-18 fluorodeoxyglucose accumulation in inflammatory pancreatic disease. Eur J Nucl Med. 1998; 25:259–264. PMID: 9580859.
Article19. Balink H, Tan SS, Veeger NJ, Holleman F, van Eck-Smit BL, Bennink RJ, et al. 18F-FDG PET/CT in inflammation of unknown origin: a cost-effectiveness pilot-study. Eur J Nucl Med Mol Imaging. 2015; 42:1408–1413. PMID: 25655485.20. Imdahl A, Nitzsche E, Krautmann F, Hogerle S, Boos S, Einert A, et al. Evaluation of positron emission tomography with 2-[18F]fluoro-2-deoxy-D-glucose for the differentiation of chronic pancreatitis and pancreatic cancer. Br J Surg. 1999; 86:194–199. PMID: 10100786.
Article21. Sperti C, Pasquali C, Decet G, Chierichetti F, Liessi G, Pedrazzoli S. F-18-fluorodeoxyglucose positron emission tomography in differentiating malignant from benign pancreatic cysts: a prospective study. J Gastrointest Surg. 2005; 9:22–28. PMID: 15623441.
Article22. Tomimaru Y, Takeda Y, Tatsumi M, Kim T, Kobayashi S, Marubashi S, et al. Utility of 2-[18F] fluoro-2-deoxy-D-glucose positron emission tomography in differential diagnosis of benign and malignant intraductal papillary-mucinous neoplasm of the pancreas. Oncol Rep. 2010; 24:613–620. PMID: 20664965.
Article23. Akita H, Takahashi H, Ohigashi H, Tomokuni A, Kobayashi S, Sugimura K, et al. FDG-PET predicts treatment efficacy and surgical outcome of pre-operative chemoradiation therapy for resectable and borderline resectable pancreatic cancer. Eur J Surg Oncol. 2017; 43:1061–1067. PMID: 28389044.
Article24. Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007; 297:267–277. PMID: 17227978.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Esophageal Leiomyoma with intense FDG uptake on 18F-FDG PET/CT
- Use of 18F-FDG PET/CT in Second Primary Cancer
- F18-fluorodeoxyglucose-positron emission tomography and computed tomography is not accurate in preoperative staging of gastric cancer
- Clinical Significance of Focal Breast Lesions Incidentally Identified by 18F-FDG PET/CT
- Usefulness of 18F-FDG PET/CT for the Evaluation of Bone Marrow Involvement in Patients with High-Grade Non-Hodgkin's Lymphoma