J Korean Med Sci.
2020 Apr;35(19):e122. 10.3346/jkms.2020.35.e122.
Development of End Stage Renal Disease after Long-Term Ingestion of Chaga Mushroom: Case Report and Review of Literature
- Affiliations
-
- 1Division of Nephrology, Department of Internal Medicine, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 2Division of Nephrology, Department of Internal Medicine, Jeju National University School of Medicine, Jeju, Korea
- 3Division of Nephrology, Department of Internal Medicine, Eunpyeong St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 4Transplant Research Center, Department of Internal Medicine, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 5Convergent Research Consortium for Immunologic Disease, Seoul St. Mary's Hospital, The Collage of Medicine, The Catholic University of Korea, Seoul, Korea
- 6Hankook Renal Pathology Lab., Seoul, Korea
- KMID: 2500628
- DOI: http://doi.org/10.3346/jkms.2020.35.e122
Abstract
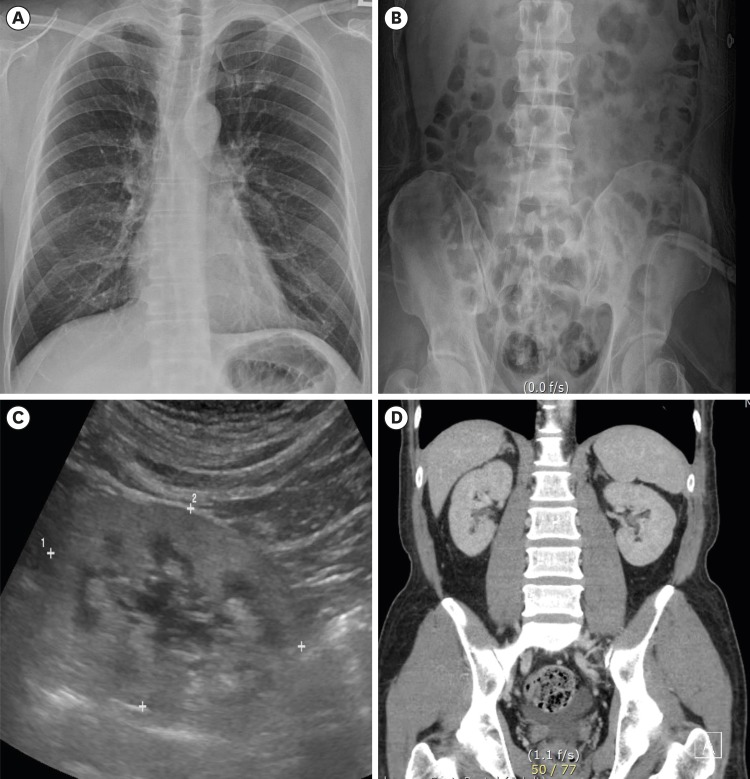
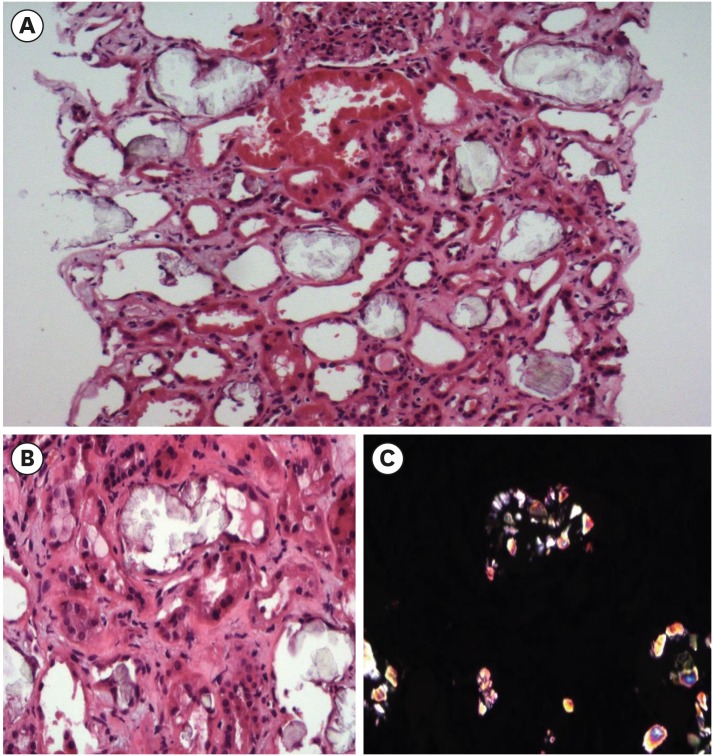
- Chaga mushrooms are widely used in folk remedies and in alternative medicine. Contrary to many beneficial effects, its adverse effect is rarely reported. We here report a case of end-stage renal disease after long-term taking Chaga mushroom. A 49-year-old Korean man with end stage renal disease (ESRD) was transferred to our hospital. Review of kidney biopsy finding was consistent with chronic tubulointerstitial nephritis with oxalate crystal deposits and drug history revealed long-term exposure to Chaga mushroom powder due to intractable atopic dermatitis. We suspected the association between Chaga mushroom and oxalate nephropathy, and measured the oxalate content of remained Chaga mushroom. The Chaga mushroom had extremely high oxalate content (14.2/100 g). Estimated daily oxalate intake of our case was 2 times for four years and 5 times for one year higher than that of usual diet. Chaga mushroom is a potential risk factor of chronic kidney disease considering high oxalate content. Nephrologist should consider oxalate nephropathy in ESRD patients exposed to Chaga mushrooms.
Figure
Reference
-
1. Kikuchi Y, Seta K, Ogawa Y, Takayama T, Nagata M, Taguchi T, et al. Chaga mushroom-induced oxalate nephropathy. Clin Nephrol. 2014; 81(6):440–444. PMID: 23149251.
Article2. Lumlertgul N, Siribamrungwong M, Jaber BL, Susantitaphong P. Secondary Oxalate Nephropathy: A Systematic Review. Kidney Int Rep. 2018; 3(6):1363–1372. PMID: 30450463.3. Sasaki M, Murakami M, Matsuo K, Matsuo Y, Tanaka S, Ono T, et al. Oxalate nephropathy with a granulomatous lesion due to excessive intake of peanuts. Clin Exp Nephrol. 2008; 12(4):305–308. PMID: 18335167.
Article4. Lewandowski S, Rodgers A, Schloss I. The influence of a high-oxalate/low-calcium diet on calcium oxalate renal stone risk factors in non-stone-forming black and white South African subjects. BJU Int. 2001; 87(4):307–311. PMID: 11251520.
Article5. Lewandowski S, Rodgers AL, Laube N, von Unruh G, Zimmermann D, Hesse A. Oxalate and its handling in a low stone risk vs a stone-prone population group. World J Urol. 2005; 23(5):330–333. PMID: 16283325.
Article6. Beier RC. Natural pesticides and bioactive components in foods. Rev Environ Contam Toxicol. 1990; 113:47–137. PMID: 2404325.
Article7. Glew RH, Sun Y, Horowitz BL, Konstantinov KN, Barry M, Fair JR, et al. Nephropathy in dietary hyperoxaluria: A potentially preventable acute or chronic kidney disease. World J Nephrol. 2014; 3(4):122–142. PMID: 25374807.
Article8. Glamočlija J, Ćirić A, Nikolić M, Fernandes Â, Barros L, Calhelha RC, et al. Chemical characterization and biological activity of Chaga (Inonotus obliquus), a medicinal “mushroom”. J Ethnopharmacol. 2015; 162:323–332. PMID: 25576897.
Article9. Shashkina MY, Shashkin PN, Sergeev AV. Chemical and medicobiological properties of chaga. [review]. Pharm Chem J. 2006; 40(10):560–568.10. Chen CL, Fang HC, Chou KJ, Wang JS, Chung HM. Acute oxalate nephropathy after ingestion of star fruit. Am J Kidney Dis. 2001; 37(2):418–422. PMID: 11157385.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Improvement of Chaga Mushroom (Inonotus Obliquus) Extract Supplementation on the Blood Glucose and Cellular DNA Damage in Streptozotocin-Induced Diabetic Rats
- Common Analgesic Agents and Their Roles in Analgesic Nephropathy: A Commentary on the Evidence
- Acquired Cystic Kidney Disease in Patients Undergoing Long-term Hemodialysis Treatment
- Hemangioma Mistaken for Renal Cell Carcinoma in a Patient With End-Stage Renal Disease: A Case Report
- A Case of Erythrocytosis in Long-term Hemodialysis Patient without Erythropoietin Therapy