Cancer Res Treat.
2020 Apr;52(2):622-633. 10.4143/crt.2019.593.
CXCL-13 Regulates Resistance to 5-Fluorouracil in Colorectal Cancer
- Affiliations
-
- 1Department of Colorectal Surgery, Sir Run Run Shaw Hospital of Zhejiang University, Hangzhou, China
- 2Zhejiang Province Key Laboratory of Biological Treatment, Hangzhou, China
- 3Key Laboratory of Regenerative Medicine of Ministry of Education, Institute of Aging and Regenerative Medicine, Jinan University, Guangzhou, China
- KMID: 2500346
- DOI: http://doi.org/10.4143/crt.2019.593
Abstract
- Purpose
5-Fluorouracil (5-Fu) is used as a conventional chemotherapy drug in chemotherapy for patients with advanced colorectal cancer, but many patients still suffer from treatment failure due to 5-Fu resistance. Emerging observations revealed the important role of chemokine (C-X-C motif) ligand 13 (CXCL-13) in tumor microenvironment and its relationship with prognosis in patients with colorectal cancer. This study is designed to reveal the important role of CXCL-13 in causing colorectal cancer resistance to 5-Fu.
Materials and Methods
CXCL-13 levels of patient's serum or cell culture supernatants were measured separately by enzyme-linked immunosorbent assay. In cell assays, cell viability is detected by Cell Counting Kit-8. Therefore, the recombinant human CXCL-13 was used to simulate its high expression in cells while its antibody and siRNA were used to reduce CXCL-13 expression in cells.
Results
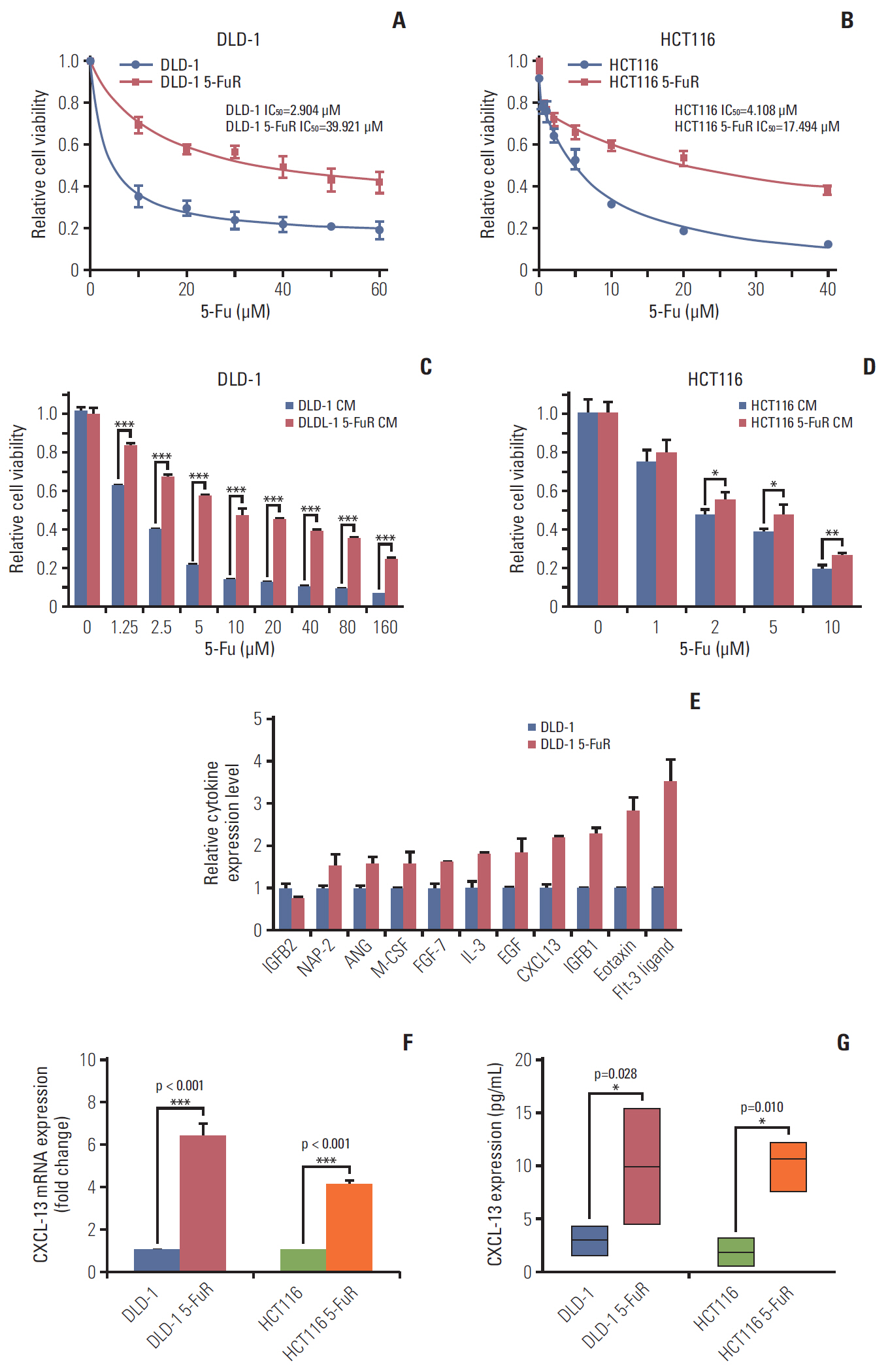
In this study, we demonstrated that CXCL-13 is associated with 5-Fu resistance by culture medium exchange experiments and cytokine arrays of colorectal cancer resistant and nonresistant cells. Clinical studies showed that CXCL-13 is highly expressed in the serum of 5-Fu–resistant patients. High levels of serum CXCL-13 also predict a worse clinical outcome. The addition of recombinant CXCL-13 cytokine resulted in 5-Fu resistance, while its antibody overcame 5-Fu resistance, and knockdown of CXCL-13 expression by siRNA also reduced 5-Fu resistance, which can be saved by added recombination CXCL-13.
Conclusion
These results not only identify a CXCL-13 mediated 5-Fu resistance mechanism but also provide a novel target for 5-Fu–resistant colorectal cancer in prevention and treatment strategies.
Figure
Reference
-
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424.
Article2. Center MM, Jemal A, Smith RA, Ward E. Worldwide variations in colorectal cancer. CA Cancer J Clin. 2009; 59:366–78.
Article3. Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000; 343:905–14.4. Panczyk M. Pharmacogenetics research on chemotherapy resistance in colorectal cancer over the last 20 years. World J Gastroenterol. 2014; 20:9775–827.
Article5. Wu Q, Yang Z, Nie Y, Shi Y, Fan D. Multi-drug resistance in cancer chemotherapeutics: mechanisms and lab approaches. Cancer Lett. 2014; 347:159–66.
Article6. Iyer AK, Singh A, Ganta S, Amiji MM. Role of integrated cancer nanomedicine in overcoming drug resistance. Adv Drug Deliv Rev. 2013; 65:1784–802.
Article7. Xie T, Huang M, Wang Y, Wang L, Chen C, Chu X. MicroRNAs as regulators, biomarkers and therapeutic targets in the drug resistance of colorectal cancer. Cell Physiol Biochem. 2016; 40:62–76.
Article8. Shen Y, Tong M, Liang Q, Guo Y, Sun HQ, Zheng W, et al. Epigenomics alternations and dynamic transcriptional changes in responses to 5-fluorouracil stimulation reveal mechanisms of acquired drug resistance of colorectal cancer cells. Pharmacogenomics J. 2018; 18:23–8.
Article9. Jones VS, Huang RY, Chen LP, Chen ZS, Fu L, Huang RP. Cytokines in cancer drug resistance: cues to new therapeutic strategies. Biochim Biophys Acta. 2016; 1865:255–65.
Article10. Zahreddine H, Borden KL. Mechanisms and insights into drug resistance in cancer. Front Pharmacol. 2013; 4:28.
Article11. Chow MT, Luster AD. Chemokines in cancer. Cancer Immunol Res. 2014; 2:1125–31.
Article12. Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004; 4:540–50.
Article13. Huber AK, Irani DN. Targeting CXCL13 during neuroinflammation. Adv Neuroimmune Biol. 2015; 6:1–8.
Article14. Gu-Trantien C, Loi S, Garaud S, Equeter C, Libin M, de Wind A, et al. CD4(+) follicular helper T cell infiltration predicts breast cancer survival. J Clin Invest. 2013; 123:2873–92.
Article15. Chen L, Huang Z, Yao G, Lyu X, Li J, Hu X, et al. The expression of CXCL13 and its relation to unfavorable clinical characteristics in young breast cancer. J Transl Med. 2015; 13:168.
Article16. Gu-Trantien C, Migliori E, Buisseret L, de Wind A, Brohee S, Garaud S, et al. CXCL13-producing TFH cells link immune suppression and adaptive memory in human breast cancer. JCI Insight. 2017; 2:91487.
Article17. Fan L, Zhu Q, Liu L, Zhu C, Huang H, Lu S, et al. CXCL13 is androgen-responsive and involved in androgen induced prostate cancer cell migration and invasion. Oncotarget. 2017; 8:53244–61.
Article18. Lazennec G, Richmond A. Chemokines and chemokine receptors: new insights into cancer-related inflammation. Trends Mol Med. 2010; 16:133–44.
Article19. Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013; 39:782–95.
Article20. Agesen TH, Sveen A, Merok MA, Lind GE, Nesbakken A, Skotheim RI, et al. ColoGuideEx: a robust gene classifier specific for stage II colorectal cancer prognosis. Gut. 2012; 61:1560–7.
Article21. Olsen RS, Nijm J, Andersson RE, Dimberg J, Wagsater D. Circulating inflammatory factors associated with worse long-term prognosis in colorectal cancer. World J Gastroenterol. 2017; 23:6212–9.
Article22. Murakami Y, Kazuno H, Emura T, Tsujimoto H, Suzuki N, Fukushima M. Different mechanisms of acquired resistance to fluorinated pyrimidines in human colorectal cancer cells. Int J Oncol. 2000; 17:277–83.
Article23. Butera G, Pacchiana R, Donadelli M. Autocrine mechanisms of cancer chemoresistance. Semin Cell Dev Biol. 2018; 78:3–12.
Article24. Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010; 29:4741–51.
Article25. Miller MA, Sullivan RJ, Lauffenburger DA. Molecular pathways: receptor ectodomain shedding in treatment, resistance, and monitoring of cancer. Clin Cancer Res. 2017; 23:623–9.
Article26. Zhou W, Sun W, Yung MMH, Dai S, Cai Y, Chen CW, et al. Autocrine activation of JAK2 by IL-11 promotes platinum drug resistance. Oncogene. 2018; 37:3981–97.
Article27. Qi XW, Xia SH, Yin Y, Jin LF, Pu Y, Hua D, et al. Expression features of CXCR5 and its ligand, CXCL13 associated with poor prognosis of advanced colorectal cancer. Eur Rev Med Pharmacol Sci. 2014; 18:1916–24.28. Lillard J, Singh R, Sharma P, Singh S. CXCL13 inhibition prevents bone metastasis in hormone-refractory prostate cancer (133.8). J Immunol. 2010; 184:133.8.29. Zhu Z, Zhang X, Guo H, Fu L, Pan G, Sun Y. CXCL13-CXCR5 axis promotes the growth and invasion of colon cancer cells via PI3K/AKT pathway. Mol Cell Biochem. 2015; 400:287–95.
Article30. Waldner MJ, Bindea G, Mlecnik B, Angell HK, Foersch S, Höpken UE, et al. 779 CXCL13-CXCR5 signaling is required for the anti-tumor immune response in colorectal cancer. Gastroenterology. 2014; 146:S131.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Phase II study of 5-fluorouracil and recombinant interferon-gamma in patients with advanced colorectal cancer
- 5-fluorouracil and low dose leucovorin in advanced colorectal carcinoma
- 5-fluorouracil and low dose leucovorin in advanced colorectal carcinoma
- 5-fluorouracil and low dose leucovorin combination chemotherapy for metastatic or recurrent colorectal cancer
- Cellular Prion Protein Enhances Drug Resistance of Colorectal Cancer Cells via Regulation of a Survival Signal Pathway