Brain Tumor Res Treat.
2020 Apr;8(1):43-52. 10.14791/btrt.2020.8.e1.
Inhibition of the Spectraplakin Protein Microtubule ActinCrosslinking Factor 1 Sensitizes Glioblastomas to Radiation
- Affiliations
-
- 1Department of Biological Sciences, Tennessee State University, Nashville, TN, USA
- KMID: 2500121
- DOI: http://doi.org/10.14791/btrt.2020.8.e1
Abstract
- Background
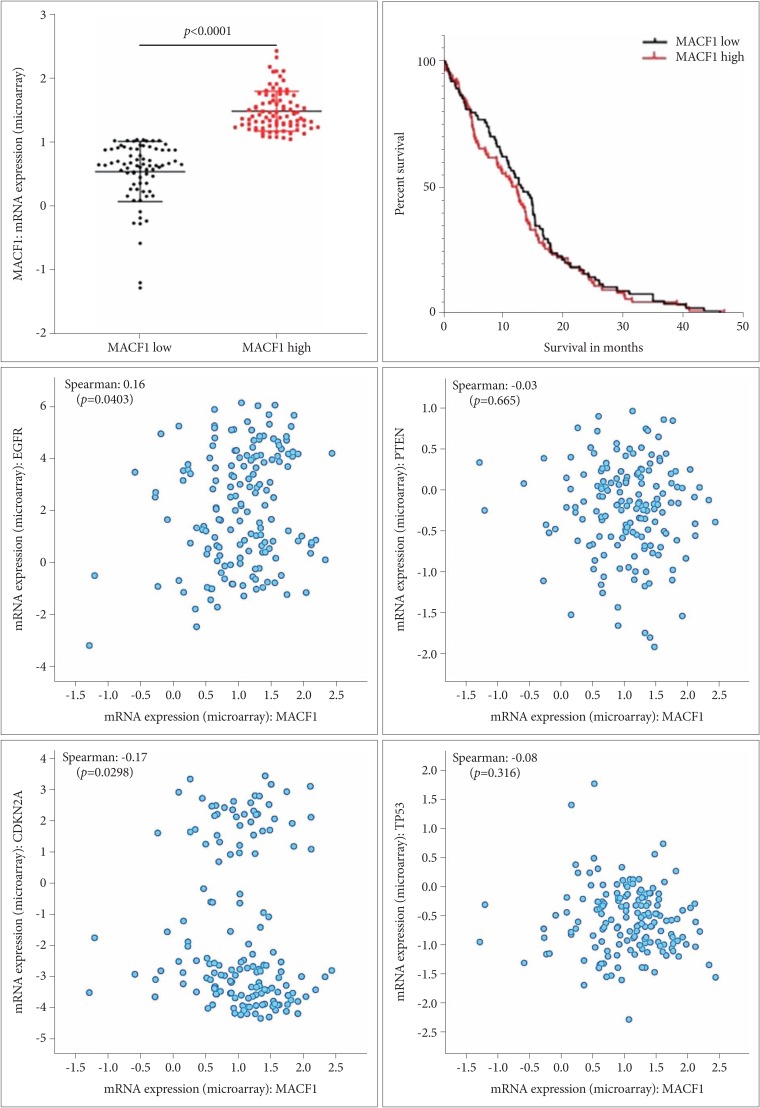
: Microtubule actin crosslinking factor 1 (MACF1) is a spectraplakin cytoskeletal crosslinking protein whose function and role in cancer biology has lacked investigation. Recent studies have identified MACF1 as a novel target in glioblastomas expressed in tissue from tumor patient explants but not normal brain tissue and when silenced has an antitumorigenic impact on these tumors. Radiation as a single agent therapy to treat glioblastomas has been used for decades and has done little to improve survival of individuals diagnosed with this disease. However, contemporary clinical radiotherapy protocols have provided evidence that combinatorial radiotherapy approaches confer a therapeutic benefit in glioblastoma patients. In this study MACF1 was investigated as a radiosensitization target in glioblastomas.
Methods
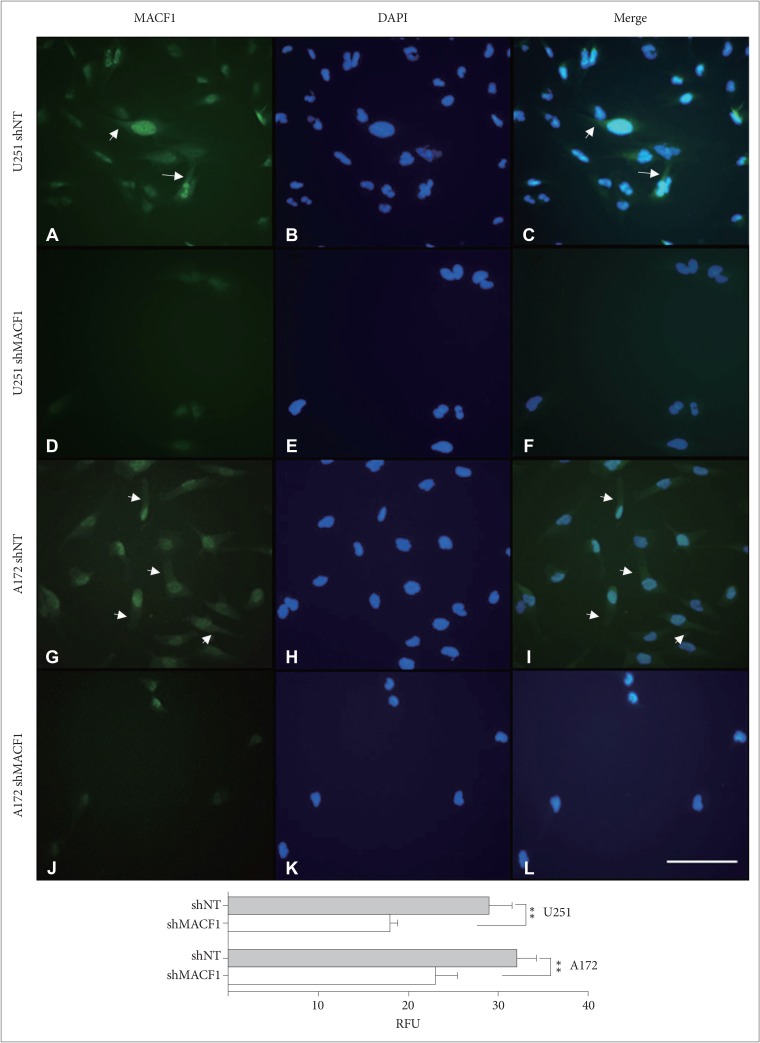
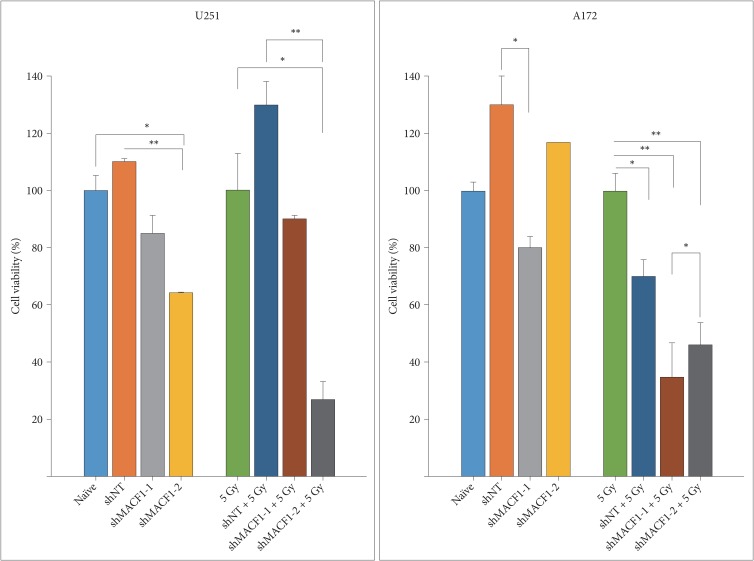
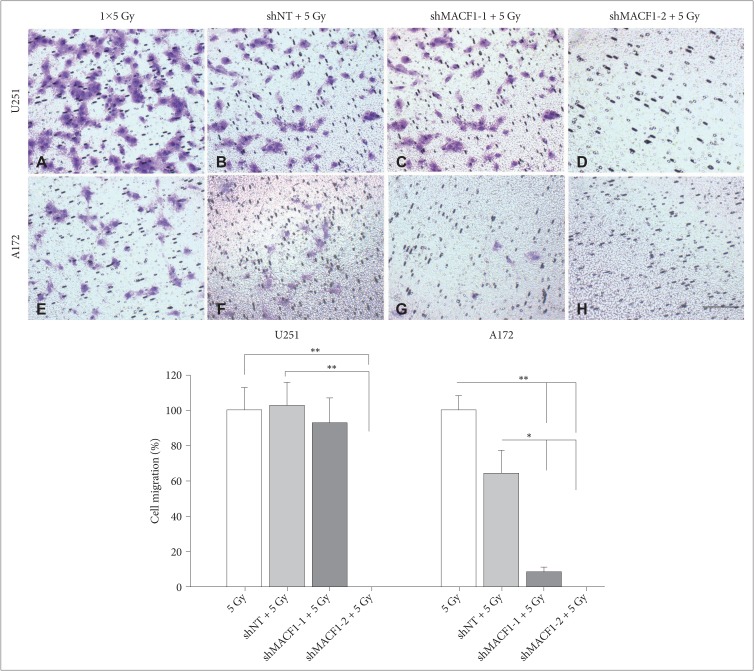
: To provide context of MACF1 in glioblastomas, The Cancer Genome Atlas expression analyses were performed in conjunction with genes associated with glioblastoma evolution, while a genetic inhibitory approach, cell migratory assays, and immunofluorescence procedures were used to evaluate responses to MACF1 suppression with radiation. Additionally, expression analyses were conducted to assess co-expression of mTOR signaling pathway regulators and MACF1 in glioblastoma patient samples.
Results
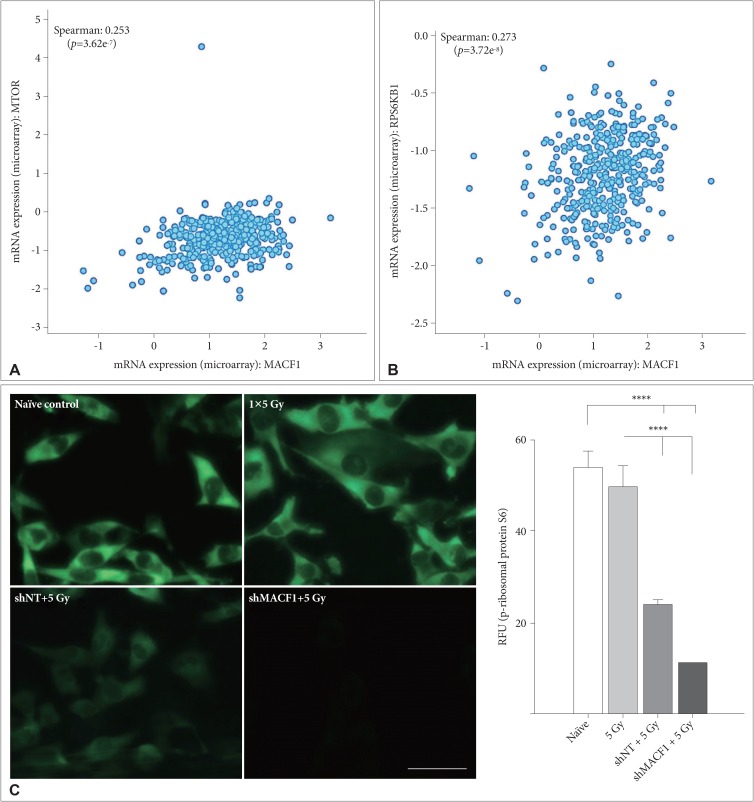
: Our amalgamation approach demonstrated that negative regulation of MACF1, which was positively correlated with epidermal growth factor receptor and p70s6k expression, enhanced the sensitivity of glioblastoma cells to radiation as a consequence of reducing glioblastoma cell viability and migration. Mechanistically, the antitumorigenic effects on glioblastoma cell behaviors after radiation and impairing MACF1 function were associated with decreased expression of ribosomal protein S6, a downstream effector of p70s6k.
Conclusion
: MACF1 represents a diagnostic marker with target specificity in glioblastomas that can enhance the efficacy of radiation while minimizing normal tissue toxicity. This approach could potentially expand combinatorial radiation strategies for glioblastoma treatments via impairment of translational regulatory processes that contribute to poor patient survival.
Keyword
Figure
Reference
-
1. Bernier G, Mathieu M, De Repentigny Y, Vidal SM, Kothary R. Cloning and characterization of mouse ACF7, a novel member of the dystonin subfamily of actin binding proteins. Genomics. 1996; 38:19–29. PMID: 8954775.2. Ka M, Jung EM, Mueller U, Kim WY. MACF1 regulates the migration of pyramidal neurons via microtubule dynamics and GSK-3 signaling. Dev Biol. 2014; 395:4–18. PMID: 25224226.3. Hu L, Su P, Li R, et al. Knockdown of microtubule actin crosslinking factor 1 inhibits cell proliferation in MC3T3-E1 osteoblastic cells. BMB Rep. 2015; 48:583–588. PMID: 26277981.4. Afghani N, Mehta T, Wang J, Tang N, Skalli O, Quick QA. Microtubule actin cross-linking factor 1, a novel target in glioblastoma. Int J Oncol. 2017; 50:310–316. PMID: 27959385.5. Arai E, Sakamoto H, Ichikawa H, et al. Multilayer-omics analysis of renal cell carcinoma, including the whole exome, methylome and transcriptome. Int J Cancer. 2014; 135:1330–1342. PMID: 24504440.6. Chang YS, Huang HD, Yeh KT, Chang JG. Identification of novel mutations in endometrial cancer patients by whole-exome sequencing. Int J Oncol. 2017; 50:1778–1784. PMID: 28339086.7. Chen HJ, Lin CM, Lin CS, Perez-Olle R, Leung CL, Liem RK. The role of microtubule actin cross-linking factor 1 (MACF1) in the Wnt signaling pathway. Genes Dev. 2006; 20:1933–1945. PMID: 16815997.8. McCord M, Mukouyama YS, Gilbert MR, Jackson S. Targeting WNT signaling for multifaceted glioblastoma therapy. Front Cell Neurosci. 2017; 11:318. PMID: 29081735.9. Shapiro WR, Green SB, Burger PC, et al. Randomized trial of three chemotherapy regimens and two radiotherapy regimens and two radiotherapy regimens in postoperative treatment of malignant glioma. Brain tumor cooperative group trial 8001. J Neurosurg. 1989; 71:1–9.10. Khosla D. Concurrent therapy to enhance radiotherapeutic outcomes in glioblastoma. Ann Transl Med. 2016; 4:54. PMID: 26904576.11. Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009; 10:459–466. PMID: 19269895.12. Kahn J, Hayman TJ, Jamal M, et al. The mTORC1/mTORC2 inhibitor AZD2014 enhances the radiosensitivity of glioblastoma stem-like cells. Neuro Oncol. 2014; 16:29–37. PMID: 24311635.13. Gil del Alcazar CR, Hardebeck MC, Mukherjee B, et al. Inhibition of DNA double-strand break repair by the dual PI3K/mTOR inhibitor NVP-BEZ235 as a strategy for radiosensitization of glioblastoma. Clin Cancer Res. 2014; 20:1235–1248. PMID: 24366691.14. Ahmed SU, Carruthers R, Gilmour L, Yildirim S, Watts C, Chalmers AJ. Selective inhibition of parallel DNA damage response pathways optimizes radiosensitization of glioblastoma stem-like cells. Cancer Res. 2015; 75:4416–4428. PMID: 26282173.15. King AR, Corso CD, Chen EM, et al. Local DNA repair inhibition for sustained radiosensitization of high-grade gliomas. Mol Cancer Ther. 2017; 16:1456–1469. PMID: 28566437.16. Shi F, Guo H, Zhang R, et al. The PI3K inhibitor GDC-0941 enhances radiosensitization and reduces chemoresistance to temozolomide in GBM cell lines. Neuroscience. 2017; 346:298–308. PMID: 28147244.17. Sulzmaier FJ, Ramos JW. RSK isoforms in cancer cell invasion and metastasis. Cancer Res. 2013; 73:6099–6105. PMID: 24097826.18. Cargnello M, Tcherkezian J, Roux PP. The expanding role of mTOR in cancer cell growth and proliferation. Mutagenesis. 2015; 30:169–176. PMID: 25688110.19. Ronellenfitsch MW, Zeiner PS, Mittelbronn M, et al. Akt and mTORC1 signaling as predictive biomarkers for the EGFR antibody nimotuzumab in glioblastoma. Acta Neuropathol Commun. 2018; 6:81. PMID: 30129426.20. Sekhar KR, Benamar M, Venkateswaran A, et al. Targeting nucleophosmin 1 represents a rational strategy for radiation sensitization. Int J Radiat Oncol Biol Phys. 2014; 89:1106–1114. PMID: 25035215.21. The cBioPortal for Cancer Genomics. The Cancer Genome Atlas. Accessed December 15, 2019. at https://www.cbioportal.org/.22. Kao GD, Jiang Z, Fernandes AM, Gupta AK, Maity A. Inhibition of phosphatidylinositol-3-OH kinase/Akt signaling impairs DNA repair in glioblastoma cells following ionizing radiation. J Biol Chem. 2007; 282:21206–21212. PMID: 17513297.23. Kil WJ, Cerna D, Burgan WE, et al. In vitro and in vivo radiosensitization induced by the DNA methylating agent temozolomide. Clin Cancer Res. 2008; 14:931–938. PMID: 18245557.24. Sheng Y, Xu M, Li C, et al. Nm23-H1 is involved in the repair of ionizing radiation-induced DNA double-strand breaks in the A549 lung cancer cell line. BMC Cancer. 2018; 18:710. PMID: 29970055.25. Martinou M, Giannopoulou E, Malatara G, Argyriou AA, Kalofonos HP, Kardamakis D. Ionizing radiation affects epidermal growth factor receptor signalling and metalloproteinase secretion in glioma cells. Cancer Genomics Proteomics. 2011; 8:33–38. PMID: 21289335.26. Contessa JN, Hampton J, Lammering G, et al. Ionizing radiation activates Erb-B receptor dependent Akt and p70 S6 kinase signaling in carcinoma cells. Oncogene. 2002; 21:4032–4041. PMID: 12037685.27. Kim Y, Kim KH, Lee J, et al. Wnt activation is implicated in glioblastoma radioresistance. Lab Invest. 2012; 92:466–473. PMID: 22083670.28. Wild-Bode C, Weller M, Rimner A, Dichgans J, Wick W. Sublethal irradiation promotes migration and invasiveness of glioma cells: implications for radiotherapy of human glioblastoma. Cancer Res. 2001; 61:2744–2750. PMID: 11289157.29. Lammering G, Hewit TH, Valerie K, et al. EGFRvIII-mediated radioresistance through a strong cytoprotective response. Oncogene. 2003; 22:5545–5553. PMID: 12944901.30. Pelloski CE, Lin E, Zhang L, et al. Prognostic associations of activated mitogen-activated protein kinase and Akt pathways in glioblastoma. Clin Cancer Res. 2006; 12:3935–3941. PMID: 16818690.31. Li B, Yuan M, Kim IA, Chang CM, Bernhard EJ, Shu HK. Mutant epidermal growth factor receptor displays increased signaling through the phosphatidylinositol-3 kinase/AKT pathway and promotes radioresistance in cells of astrocytic origin. Oncogene. 2004; 23:4594–4602. PMID: 15077177.32. Liu Q, Nguyen DH, Dong Q, et al. Molecular properties of CD133+ glioblastoma stem cells derived from treatment-refractory recurrent brain tumors. J Neurooncol. 2009; 94:1–19. PMID: 19468690.33. Shih HA, Betensky RA, Dorfman MV, Louis DN, Loeffler JS, Batchelor TT. Genetic analyses for predictors of radiation response in glioblastoma. Int J Radiat Oncol Biol Phys. 2005; 63:704–710. PMID: 15978739.34. Barker FG 2nd, Simmons ML, Chang SM, et al. EGFR overexpression and radiation response in glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2001; 51:410–418. PMID: 11567815.35. Yang P, Zhang W, Wang Y, et al. IDH mutation and MGMT promoter methylation in glioblastoma: results of a prospective registry. Oncotarget. 2015; 6:40896–40906. PMID: 26503470.36. Tini P, Nardone V, Pastina P, et al. Patients affected by unmethylated O(6)-methylguanine-DNA methyltransferase glioblastoma undergoing radiochemotherapy may benefit from moderately dose-escalated radiotherapy. Biomed Res Int. 2017; 2017:9461402. PMID: 29159183.37. Miki S, Imamichi S, Fujimori H, et al. Concomitant administration of radiation with eribulin improves the survival of mice harboring intracerebral glioblastoma. Cancer Sci. 2018; 109:2275–2285. PMID: 29758120.38. Wang Y, Mei H, Shao Q, Wang J, Lin Z. Association of ribosomal protein S6 kinase 1 with cellular radiosensitivity of non-small lung cancer. Int J Radiat Biol. 2017; 93:581–589. PMID: 28276898.39. Lee S, Nahm M, Lee M, et al. The F-actin-microtubule crosslinker Shot is a platform for Krasavietz-mediated translational regulation of midline axon repulsion. Development. 2007; 134:1767–1777. PMID: 17409115.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Disruption of Microtubules Sensitizes the DNA Damage-induced Apoptosis Through Inhibiting Nuclear Factor kappaB (NF-kappaB) DNA-binding Activity
- p53 Mutation and Epidermal Growth Factor Receptor Overexpression in Glioblastoma
- Immunohistochemical Classification of Primary and Secondary Glioblastomas
- Alterations of 9p21-22 Region Encoding Genes in Primary Glioblastomas
- Kinesin Spindle Protein Inhibition in Translational Research