Korean J Radiol.
2020 Feb;21(2):181-191. 10.3348/kjr.2019.0446.
Preoperative Cardiac Computed Tomography Characteristics Associated with Recurrent Aortic Regurgitation after Aortic Valve Re-Implantation
- Affiliations
-
- 1Department of Radiology and Research Institute of Radiology, Cardiac Imaging Center, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. radkoo@amc.seoul.kr
- 2Division of Cardiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 3Department of Cardiovascular Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 2471635
- DOI: http://doi.org/10.3348/kjr.2019.0446
Abstract
OBJECTIVE
To identify the preoperative cardiac computed tomography (CT) factors influencing postoperative recurrent aortic regurgitation (AR) in patients who underwent aortic valve repair with the re-implantation technique (David operation) due to AR.
MATERIALS AND METHODS
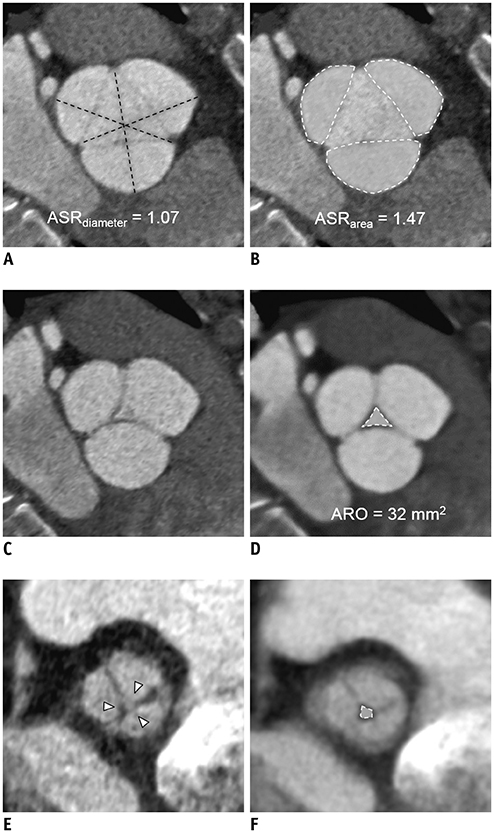
A total of 117 patients (age, 49.4 ± 15.6 years; 83 males) who underwent the David operation for AR were included in this retrospective study. Aortic root profiles including the aortic regurgitant orifice area (ARO) and the aortic cusp asymmetry ratio of the areas (ASR(area)), which is defined as the maximum/minimum areas among the three cusp areas at the level of the commissures, were measured on preoperative cardiac CT scans. Clinical and CT findings were compared between a group with recurrent AR grade < 3 (no, trivial, or mild AR) and recurrent ≥ 3 + AR. To determine the optimal cut-off values of ASR and ARO, the receiver operating characteristic (ROC) curve was used. Cox regression analysis was used for the analysis of the factors affecting recurrent 3 + AR.
RESULTS
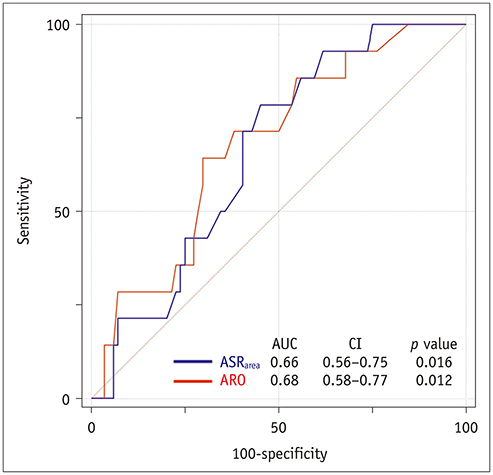
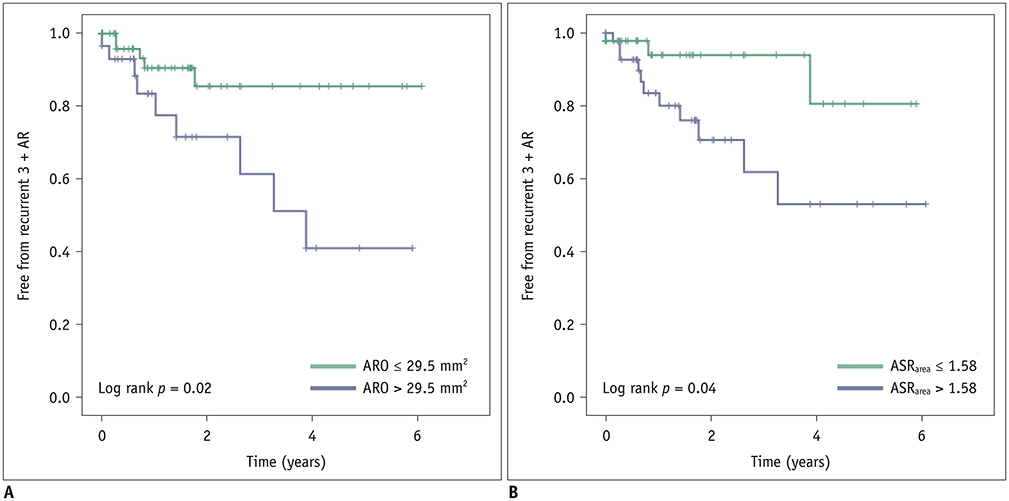
Postoperatively, recurrent 3 + AR developed in 17 (14.5%) patients and occurred within a median of 268 days (interquartile range: 78-582 days). The cut-off ARO value for discriminating the patients with recurrent 3 + AR was > 24 mm² (sensitivity, 76.5%; specificity 64.8%), and the area under the ROC curve (AUC) was 0.72. For ASR(area), the cut-off value was > 1.58 (sensitivity, 76.5%; specificity, 58.0%) and the AUC was 0.64. Multivariable Cox regression showed that ARO > 24 mm² (hazard ratio = 3.79, p = 0.020) was a potential independent parameter for recurrent 3 + AR. ROC for the linear regression model showed that the AUC for both ARO and ASR(area) was 0.73 (95% confidence interval, 0.64-0.81, p < 0.001).
CONCLUSION
ARO and ASR(area) detected on preoperative cardiac CT would be potentially helpful for identifying AR patients who may benefit from the David operation.
MeSH Terms
Figure
Reference
-
1. Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Barón-Esquivias G, Baumgartner H, et al. ESC Committee for Practice Guidelines (CPG). Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC). European Association for Cardio-Thoracic Surgery (EACTS). Guidelines on the management of valvular heart disease (version 2012): the joint task force on the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur J Cardiothorac Surg. 2012; 42:S1–S44.2. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Fleisher LA, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. 2017; 70:252–289.3. Rahimtoola SH. Choice of prosthetic heart valve for adult patients. J Am Coll Cardiol. 2003; 41:893–904.
Article4. Hammermeister K, Sethi GK, Henderson WG, Grover FL, Oprian C, Rahimtoola SH. Outcomes 15 years after valve replacement with a mechanical versus a bioprosthetic valve: final report of the Veterans Affairs randomized trial. J Am Coll Cardiol. 2000; 36:1152–1158.
Article5. Oxenham H, Bloomfield P, Wheatley DJ, Lee RJ, Cunningham J, Prescott RJ, et al. Twenty year comparison of a Bjork-Shiley mechanical heart valve with porcine bioprostheses. Heart. 2003; 89:715–721.
Article6. El Khoury G, de Kerchove L. Principles of aortic valve repair. J Thorac Cardiovasc Surg. 2013; 145:3 Suppl. S26–S29.
Article7. Langer F, Aicher D, Kissinger A, Wendler O, Lausberg H, Fries R, et al. Aortic valve repair using a differentiated surgical strategy. Circulation. 2004; 110:11 Suppl 1. II67–II73.
Article8. Minakata K, Schaff HV, Zehr KJ, Dearani JA, Daly RC, Orszulak TA, et al. Is repair of aortic valve regurgitation a safe alternative to valve replacement? J Thorac Cardiovasc Surg. 2004; 127:645–653.
Article9. Carr JA, Savage EB. Aortic valve repair for aortic insufficiency in adults: a contemporary review and comparison with replacement techniques. Eur J Cardiothorac Surg. 2004; 25:6–15.
Article10. Aicher D, Fries R, Rodionycheva S, Schmidt K, Langer F, Schäfers HJ. Aortic valve repair leads to a low incidence of valve-related complications. Eur J Cardiothorac Surg. 2010; 37:127–132.
Article11. le Polain de Waroux JB, Pouleur AC, Goffinet C, Vancraeynest D, Van Dyck M, Robert A, et al. Functional anatomy of aortic regurgitation: accuracy, prediction of surgical repairability, and outcome implications of transesophageal echocardiography. Circulation. 2007; 116:11 Suppl. I264–I269.
Article12. Boodhwani M, de Kerchove L, Glineur D, Poncelet A, Rubay J, Astarci P, et al. Repair-oriented classification of aortic insufficiency: impact on surgical techniques and clinical outcomes. J Thorac Cardiovasc Surg. 2009; 137:286–294.
Article13. de Meester C, Pasquet A, Gerber BL, Vancraeynest D, Noirhomme P, El Khoury G, et al. Valve repair improves the outcome of surgery for chronic severe aortic regurgitation: a propensity score analysis. J Thorac Cardiovasc Surg. 2014; 148:1913–1920.
Article14. Koo HJ, Kang JW, Kim JA, Kim JB, Jung SH, Choo SJ, et al. Functional classification of aortic regurgitation using cardiac computed tomography: comparison with surgical inspection. Int J Cardiovasc Imaging. 2018; 34:1295–1303.
Article15. Perry GJ, Helmcke F, Nanda NC, Byard C, Soto B. Evaluation of aortic insufficiency by Doppler color flow mapping. J Am Coll Cardiol. 1987; 9:952–959.
Article16. Lu TL, Huber CH, Rizzo E, Dehmeshki J, von Segesser LK, Qanadli SD. Ascending aorta measurements as assessed by ECG-gated multi-detector computed tomography: a pilot study to establish normative values for transcatheter therapies. Eur Radiol. 2009; 19:664–669.
Article17. David TE. Aortic valve sparing operations: outcomes at 20 years. Ann Cardiothorac Surg. 2013; 2:24–29.18. David TE, Feindel CM. An aortic valve-sparing operation for patients with aortic incompetence and aneurysm of the ascending aorta. J Thorac Cardiovasc Surg. 1992; 103:617–621. discussion 622.
Article19. le Polain de Waroux JB, Pouleur AC, Robert A, Pasquet A, Gerber BL, Noirhomme P, et al. Mechanisms of recurrent aortic regurgitation after aortic valve repair: predictive value of intraoperative transesophageal echocardiography. JACC Cardiovasc Imaging. 2009; 2:931–939.20. Stephens EH, Liang DH, Kvitting JPE, Kari FA, Fischbein MP, Mitchell RS, et al. Incidence and progression of mild aortic regurgitation after Tirone David reimplantation valve-sparing aortic root replacement. J Thorac Cardiovasc Surg. 2014; 147:169–178.e3.
Article21. Yang DH, Kim DH, Handschumacher MD, Levine RA, Kim JB, Sun BJ, et al. In vivo assessment of aortic root geometry in normal controls using 3D analysis of computed tomography. Eur Heart J Cardiovasc Imaging. 2017; 18:780–786.
Article22. Choo SJ, McRae G, Olomon JP, St George G, Davis W, Burleson-Bowles CL, et al. Aortic root geometry: pattern of differences between leaflets and sinuses of Valsalva. J Heart Valve Dis. 1999; 8:407–415.23. Kim DH, Handschumacher MD, Levine RA, Sun BJ, Jang JY, Yang DH, et al. Aortic valve adaptation to aortic root dilatation: insights into the mechanism of functional aortic regurgitation from 3-dimensional cardiac computed tomography. Circ Cardiovasc Imaging. 2014; 7:828–835.24. Di Franco A, Rong LQ, Munjal M, Weinsaft JW, Kim J, Sturla F, et al. Aortic symmetry index: initial validation of a novel preoperative predictor of recurrent aortic insufficiency after valve-sparing aortic root reconstruction. J Thorac Cardiovasc Surg. 2018; 156:1393–1394.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recent updates in transcatheter aortic valve implantation
- Congenital Quadricuspid Aortic Valve Disease
- Computed Tomography and Magnetic Resonance Imaging Findings of Bicuspid Aortic Valve and Related Abnormalities of the Heart and Thoracic Aorta
- Avulsion of Aortic Commissure: Rare Cause of Aortic Regurgitation: 2 case reports
- Congenital Quadricuspid Aortic Valve