Ann Dermatol.
2020 Apr;32(2):101-108. 10.5021/ad.2020.32.2.101.
Exploratory Study of Epidermis, Basement Membrane Zone, Upper Dermis Alterations and Wnt Pathway Activation in Melasma Compared to Adjacent and Retroauricular Skin
- Affiliations
-
- 1Department of Dermatology and Radioterapy, Botucatu School of Medicine, São Paulo State University, Botucatu, Brazil. heliomiot@fmb.unesp.br
- 2Department of Patology, Botucatu School of Medicine, São Paulo State University, Botucatu, Brazil.
- KMID: 2471335
- DOI: http://doi.org/10.5021/ad.2020.32.2.101
Abstract
- BACKGROUND
Melasma is a chronic acquired focal hypermelanosis which pathogenesis has not been fully elucidated. Classical pathophysiologic studies have analysed the affected and perilesional areas, but little is known about the status of sun-protected skin, which is subjected to the same endogenous and genetic factors.
OBJECTIVE
To assess the histological characteristics of melasma compared to adjacent and retroauricular skin.
METHODS
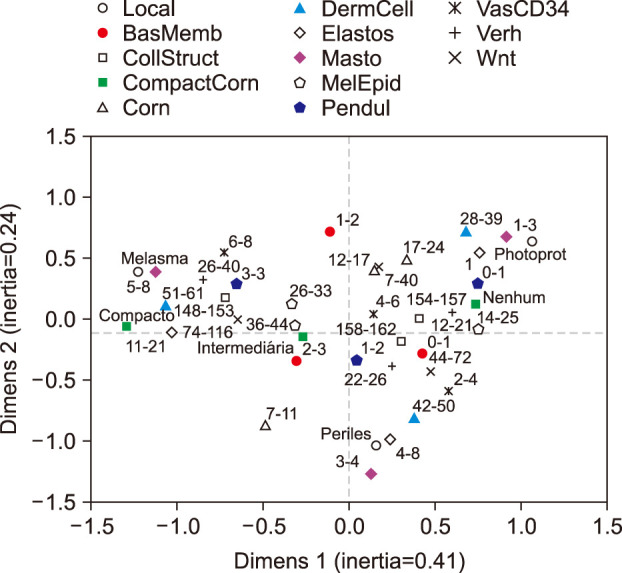
Skin samples were collected from 10 female from: melasma, perilesional area and retroauricular. The samples were stained (haematoxylin-eosin, periodic acid-Schiff, Fontana-Masson, picrosirius red, toluidine blue and Verhoeff), immunolabelled for CD34 and Wnt1. The data from the skin sites were analysed simultaneously by a multivariate model.
RESULTS
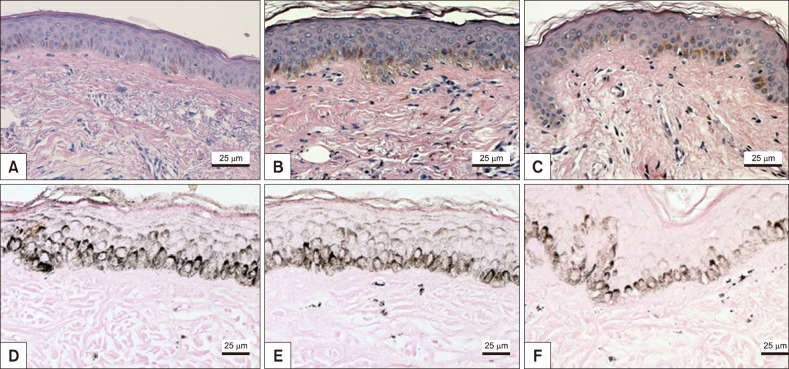
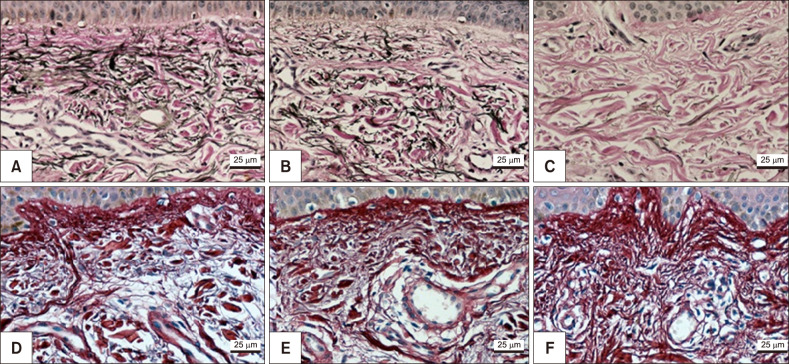
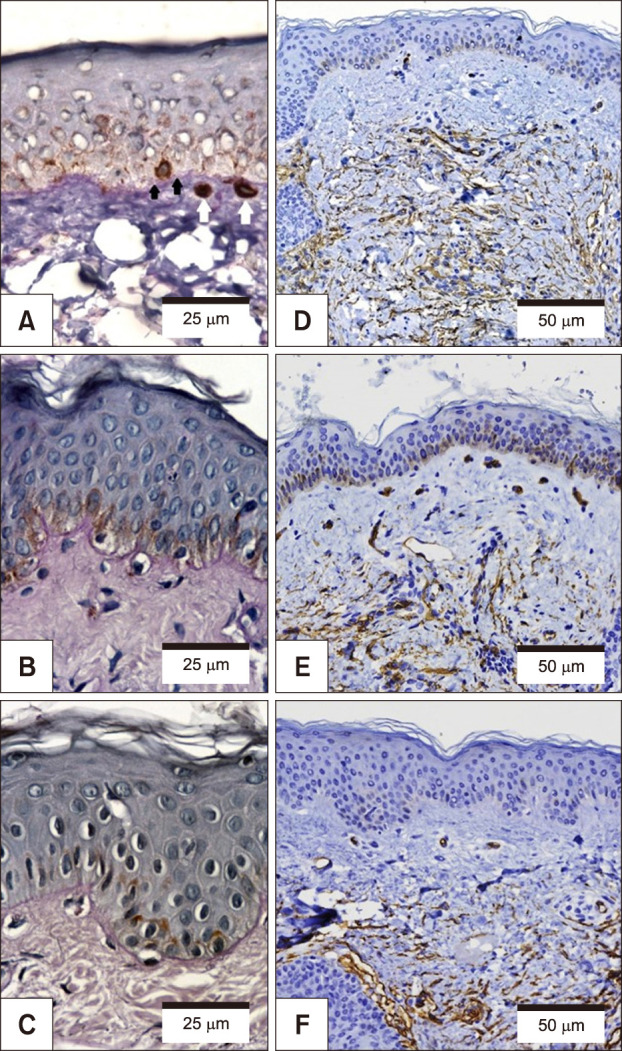
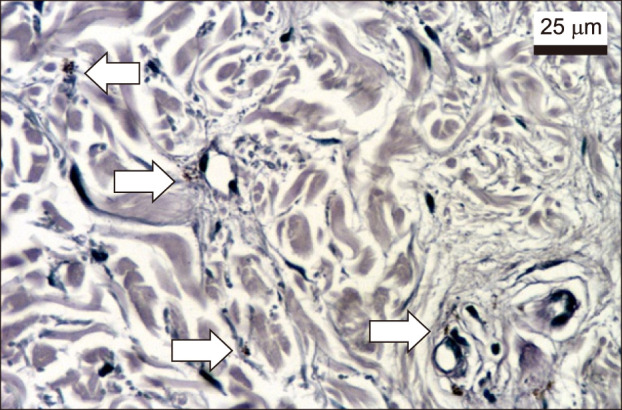
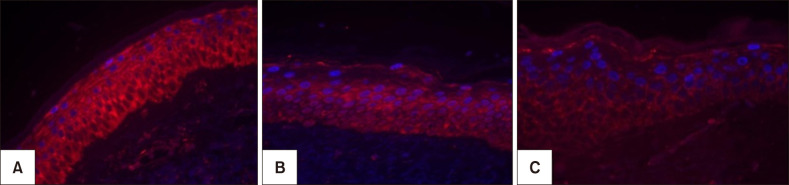
Melasma skin exhibited noteworthy stratum corneum compaction, greater collagen heterogeneity, solar elastosis, higher number of mast cells, basement membrane zone (BMZ) damage, Wnt1 expression, pendulum melanocytes, higher cellularity and vascular proliferation at the superficial dermis. Stratum corneum compaction, collagen heterogeneity and BMZ abnormalities were variables associated to melasma that not follow a continuum through retroauricular to adjacent skin. Mast cell count was the variable that disclosed correlation with the most other abnormalities as well as had the greater contribution in the multivariate model.
CONCLUSION
In addition to melanocyte hyperactivity, melasma skin exhibits alterations in the epidermal barrier, upper dermis and BMZ, which differ from the adjacent sun-exposed skin and retroauricular skin, indicating a distinct phenotype, rather than a mere extension of photoageing or intrinsic ageing. Mast cells appear to play a central role in the physiopathology of melasma.
Keyword
MeSH Terms
Figure
Reference
-
1. Maranzatto CF, Miot HA, Miot LD, Meneguin S. Psychometrican analysis and dimensional structure of the Brazilian version of melasma quality of life scale (MELASQoL-BP). An Bras Dermatol. 2016; 91:422–428. PMID: 27579735.
Article2. Holmo NF, Ramos GB, Salomão H, Werneck RI, Mira MT, Miot LDB, et al. Complex segregation analysis of facial melasma in Brazil: evidence for a genetic susceptibility with a dominant pattern of segregation. Arch Dermatol Res. 2018; 310:827–831. PMID: 30167816.
Article3. D'Elia MP, Brandão MC, de Andrade Ramos BR, da Silva MG, Miot LD, Dos Santos SE, et al. African ancestry is associated with facial melasma in women: a cross-sectional study. BMC Med Genet. 2017; 18:17. PMID: 28212612.4. Kwon SH, Hwang YJ, Lee SK, Park KC. Heterogeneous pathology of melasma and its clinical implications. Int J Mol Sci. 2016; 17:E824. PMID: 27240341.
Article5. Brianezi G, Handel AC, Schmitt JV, Miot LD, Miot HA. Changes in nuclear morphology and chromatin texture of basal keratinocytes in melasma. J Eur Acad Dermatol Venereol. 2015; 29:809–812. PMID: 24629163.
Article6. Miot HA. Sample size in clinical and experimental trials. J Vasc Bras. 2011; 10:275–278.7. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012; 9:671–675. PMID: 22930834.
Article8. Miot HA. Assessing normality of data in clinical and experimental trials. J Vasc Bras. 2017; 16:88–91. PMID: 29930631.9. Sourial N, Wolfson C, Zhu B, Quail J, Fletcher J, Karunananthan S, et al. Correspondence analysis is a useful tool to uncover the relationships among categorical variables. J Clin Epidemiol. 2010; 63:638–646. PMID: 19896800.
Article10. Miot HA. Correlation analysis in clinical and experimental studies. J Vasc Bras. 2018; 17:275–279. PMID: 30787944.11. Miot LD, Miot HA, Polettini J, Silva MG, Marques ME. Morphologic changes and the expression of alpha-melanocyte stimulating hormone and melanocortin-1 receptor in melasma lesions: a comparative study. Am J Dermatopathol. 2010; 32:676–682. PMID: 20534990.
Article12. Sarkar R, Arora P, Garg VK, Sonthalia S, Gokhale N. Melasma update. Indian Dermatol Online J. 2014; 5:426–435. PMID: 25396123.
Article13. Bhawan J, Andersen W, Lee J, Labadie R, Solares G. Photoaging versus intrinsic aging: a morphologic assessment of facial skin. J Cutan Pathol. 1995; 22:154–159. PMID: 7560349.
Article14. Espósito ACC, Brianezi G, de Souza NP, Miot LDB, Marques MEA, Miot HA. Exploring pathways for sustained melanogenesis in facial melasma: an immunofluorescence study. Int J Cosmet Sci. 2018; 40:420–424. PMID: 29846953.
Article15. Bacharach-Buhles M, Lubowietzki M, Altmeyer P. Dose-dependent shift of apoptotic and unaltered melanocytes into the dermis after irradiation with UVA 1. Dermatology. 1999; 198:5–10. PMID: 10026394.
Article16. Imokawa G, Yada Y, Morisaki N, Kimura M. Biological characterization of human fibroblast-derived mitogenic factors for human melanocytes. Biochem J. 1998; 330 Pt 3:1235–1239. PMID: 9494091.
Article17. Yoshida M, Takahashi Y, Inoue S. Histamine induces melanogenesis and morphologic changes by protein kinase A activation via H2 receptors in human normal melanocytes. J Invest Dermatol. 2000; 114:334–342. PMID: 10651995.
Article18. Hernández-Barrera R, Torres-Alvarez B, Castanedo-Cazares JP, Oros-Ovalle C, Moncada B. Solar elastosis and presence of mast cells as key features in the pathogenesis of melasma. Clin Exp Dermatol. 2008; 33:305–308. PMID: 18419607.
Article19. Iddamalgoda A, Le QT, Ito K, Tanaka K, Kojima H, Kido H. Mast cell tryptase and photoaging: possible involvement in the degradation of extra cellular matrix and basement membrane proteins. Arch Dermatol Res. 2008; 300 Suppl 1:S69–S76. PMID: 17968569.
Article20. Espósito ACC, Brianezi G, de Souza NP, Santos DC, Miot LDB, Miot HA. Ultrastructural characterization of damage in the basement membrane of facial melasma. Arch Dermatol Res. 2019; DOI: 10.1007/s00403-019-01979-w. [Epub ahead of print].
Article21. Krämer M, Sachsenmaier C, Herrlich P, Rahmsdorf HJ. UV irradiation-induced interleukin-1 and basic fibroblast growth factor synthesis and release mediate part of the UV response. J Biol Chem. 1993; 268:6734–6741. PMID: 8454646.
Article22. Kim EH, Kim YC, Lee ES, Kang HY. The vascular characteristics of melasma. J Dermatol Sci. 2007; 46:111–116. PMID: 17363223.
Article23. Kim EJ, Park HY, Yaar M, Gilchrest BA. Modulation of vascular endothelial growth factor receptors in melanocytes. Exp Dermatol. 2005; 14:625–633. PMID: 16026585.
Article24. Regazzetti C, De Donatis GM, Ghorbel HH, Cardot-Leccia N, Ambrosetti D, Bahadoran P, et al. Endothelial Cells promote pigmentation through endothelin receptor B activation. J Invest Dermatol. 2015; 135:3096–3104. PMID: 26308584.
Article25. Whyte JL, Smith AA, Helms JA. Wnt signaling and injury repair. Cold Spring Harb Perspect Biol. 2012; 4:a008078. PMID: 22723493.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Unusual Cutaneous Manifestations of Connective Tissue Diseases: IV . Bullous Eruptions in Systemic Lupus Erythematosus
- A Case of Epidermolysis Bullosa Simplex
- Immunohistochemical Demonstration of the Skin Basement Membrane Antigens by the AMex ( Acetone , Methyl Benzoate and Xylene ) Method
- Expression of Epidermal Protein Antigens and Basement Membrane Components in Human Epidermis Reconstructed by Culture
- Immune Reactions in Pterygium