Ann Hepatobiliary Pancreat Surg.
2020 Feb;24(1):52-56. 10.14701/ahbps.2020.24.1.52.
Prognostic value of COL6A3 in pancreatic adenocarcinoma
- Affiliations
-
- 1Department of Surgery, AMEOS Klinikum, Halberstadt, Germany. xristos_svor@yahoo.gr
- 2First Department of Surgery, Aristotle University of Thessaloniki, Thessaloniki, Greece.
- 3St Mary's Hospital Imperial College Healthcare NSH Trust, London, UK.
- 4Unit of Pancreatic Surgery, Interbalkan Center, Thessaloniki, Greece.
- KMID: 2471189
- DOI: http://doi.org/10.14701/ahbps.2020.24.1.52
Abstract
- BACKGROUNDS/AIMS
Pancreatic cancer is one of the most fatal human malignancies with poor prognosis, despite advances in therapy. Here, we evaluated the potential role of collagen type VI α3 chain (COL6A3) as a non-invasive biomarker for pancreatic adenocarcinoma.
METHODS
In this study, we investigated immunohistochemically the expression of COL6A3 in 30 patients with resectable pancreatic adenocarcinoma by immunohistochemistry in a tissue sample of the cancer and a tissue sample of normal pancreas for each patient. Also, we looked for associations between COL6A3 and other prognostic factors of pancreatic cancer.
RESULTS
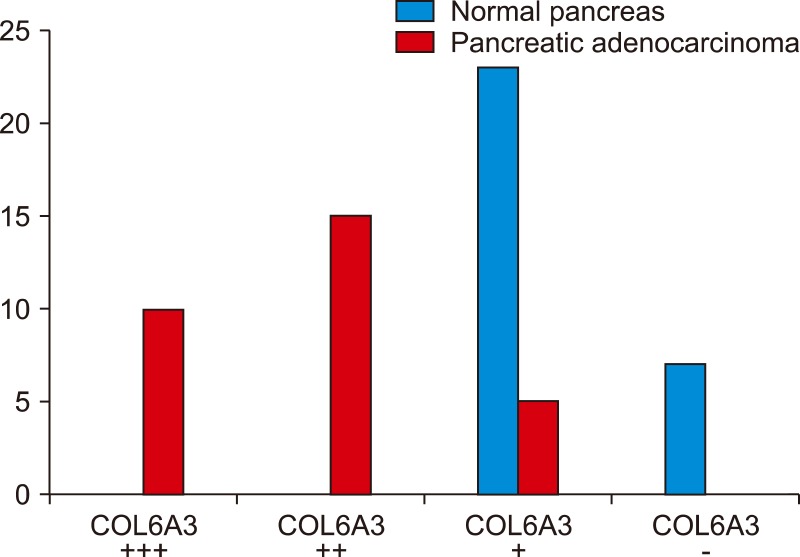
All of the pancreatic cancer tissue samples revealed in different ranges of intensity from weak (+) in 16.67%, moderate (+2) in 50%, to strongly positive (+3) in 33.33% staining for COL6A3. We found no moderate or strongly positive staining in normal pancreatic tissue. There was only weak positive staining in 23 samples (76.67%) and 7 (23.30%) were negative. Also, there was significant correlation between COL6A3 moderate and strongly expression and negative prognostic factors for pancreatic cancer.
CONCLUSIONS
The greatest density of COL6A3 was observed in pancreatic cancer tissues and was correlated with negative prognostic factors for pancreatic cancer. Therefore, we suggest that COL6A3 could be used as prognostic factor in pancreatic cancer, but more studies need to prove its value.
Keyword
MeSH Terms
Figure
Reference
-
1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017; 67:7–30. PMID: 28055103.
Article2. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90. PMID: 21296855.
Article3. Rucki AA, Zheng L. Pancreatic cancer stroma: understanding biology leads to new therapeutic strategies. World J Gastroenterol. 2014; 20:2237–2246. PMID: 24605023.
Article4. Neesse A, Bauer CA, Öhlund D, Lauth M, Buchholz M, Michl P, et al. Stromal biology and therapy in pancreatic cancer: ready for clinical translation. Gut. 2019; 68:159–171. PMID: 30177543.
Article5. Weniger M, Honselmann KC, Liss AS. The extracellular matrix and pancreatic cancer: a complex relationship. Cancers (Basel). 2018; 10:E316. PMID: 30200666.
Article6. Llacua LA, Hoek A, de Haan BJ, de Vos P. Collagen type VI interaction improves human islet survival in immunoisolating microcapsules for treatment of diabetes. Islets. 2018; 10:60–68. PMID: 29521546.
Article7. Zazuli Z, Barliana MI, Mulyani UA, Perwitasari DA, Ng H, Abdulah R. Polymorphism of PXR gene associated with the increased risk of drug-induced liver injury in Indonesian pulmonary tuberculosis patients. J Clin Pharm Ther. 2015; 40:680–684. PMID: 26417664.
Article8. Moon JY, Chang BC, Lee KE, Bang JS, Gwak HS. Effects of pregnane X receptor genetic polymorphisms on stable warfarin doses. J Cardiovasc Pharmacol Ther. 2015; 20:532–538. PMID: 25848132.
Article9. Liu W, Li L, Ye H, Tao H, He H. Role of COL6A3 in colorectal cancer. Oncol Rep. 2018; 39:2527–2536. PMID: 29620224.
Article10. Duan Y, Liu G, Sun Y, Wu J, Xiong Z, Jin T, et al. COL6A3 polymorphisms were associated with lung cancer risk in a Chinese population. Respir Res. 2019; 20:143. PMID: 31286980.
Article11. Xie X, Liu X, Zhang Q, Yu J. Overexpression of collagen VI α3 in gastric cancer. Oncol Lett. 2014; 7:1537–1543. PMID: 24765172.
Article12. American Cancer Society. American Joint Committee on Cancer (AJCC) TNM staging system. New York: American Cancer Society;2016. cited 2017 Jan 4. Available from: http://www.cancer.org/cancer/pancreaticcancer/detailedguide/pancreatic-cancer-staging.13. Lin R, Han CQ, Wang WJ, Liu J, Qian W, Ding Z, et al. Analysis on survival and prognostic factors in patients with resectable pancreatic adenocarcinoma. J Huazhong Univ Sci Technolog Med Sci. 2017; 37:612–620. PMID: 28786050.
Article14. Yamamoto T, Yagi S, Kinoshita H, Sakamoto Y, Okada K, Uryuhara K, et al. Long-term survival after resection of pancreatic cancer: a single-center retrospective analysis. World J Gastroenterol. 2015; 21:262–268. PMID: 25574100.
Article15. Vernerey D, Huguet F, Vienot A, Goldstein D, Paget-Bailly S, Van Laethem JL, et al. Prognostic nomogram and score to predict overall survival in locally advanced untreated pancreatic cancer (PROLAP). Br J Cancer. 2016; 115:281–289. PMID: 27404456.
Article16. Wang XD, Qian JJ, Bai DS, Li ZN, Jiang GQ, Yao J. Marital status independently predicts pancreatic cancer survival in patients treated with surgical resection: an analysis of the SEER database. Oncotarget. 2016; 7:24880–24887. PMID: 27036036.
Article17. Butturini G, Stocken DD, Wente MN, Jeekel H, Klinkenbijl JH, Bakkevold KE, et al. Pancreatic Cancer Meta-Analysis Group. Influence of resection margins and treatment on survival in patients with pancreatic cancer: meta-analysis of randomized controlled trials. Arch Surg. 2008; 143:75–83. PMID: 18209156.18. Pawlik TM, Gleisner AL, Cameron JL, Winter JM, Assumpcao L, Lillemoe KD, et al. Prognostic relevance of lymph node ratio following pancreaticoduodenectomy for pancreatic cancer. Surgery. 2007; 141:610–618. PMID: 17462460.
Article19. Asari S, Matsumoto I, Toyama H, Shinzeki M, Goto T, Ishida J, et al. Preoperative independent prognostic factors in patients with borderline resectable pancreatic ductal adenocarcinoma following curative resection: the neutrophil-lymphocyte and platelet-lymphocyte ratios. Surg Today. 2016; 46:583–592. PMID: 26108488.
Article20. Yu J, Ding Z, Yang Y, Liu S. Increased platelet-to-lymphocytes ratio is associated with poor long-term prognosis in patients with pancreatic cancer after surgery. Medicine (Baltimore). 2018; 97:e11002. PMID: 29923983.
Article21. Li W, Tao L, Zhang L, Xiu D. Prognostic role of lymphocyte to monocyte ratio for patients with pancreatic cancer: a systematic review and meta-analysis. Onco Targets Ther. 2017; 10:3391–3397. PMID: 28744143.
Article22. La Torre M, Nigri G, Cavallini M, Mercantini P, Ziparo V, Ramacciato G. The glasgow prognostic score as a predictor of survival in patients with potentially resectable pancreatic adenocarcinoma. Ann Surg Oncol. 2012; 19:2917–2923. PMID: 22488099.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prognostic Factors and Survival Rate of Pancreatic Adenocarcinoma after Curative Surgery
- A Case of Mucinous Cystic Adenocarcinoma of the Pancreas
- Clinical importance of preoperative and postoperative prognostic nutritional index in patients with pancreatic ductal adenocarcinoma
- Subcutaneous Fat Necrosis Associated with Pancreatic Adenocarcinoma: A case report
- A Case of Cutaneous Metastasis from Pancreatic Adenocarcinoma