Korean J Transplant.
2019 Dec;33(4):146-152. 10.4285/jkstn.2019.33.4.146.
Progression of diabetic nephropathy after successful pancreas transplantation alone: a case report
- Affiliations
-
- 1Department of Surgery, Pusan National University Yangsan Hospital, Pusan National University School of Medicine, Yangsan, Korea. gmoolpop@gmail.com
- 2Department of Pathology, Pusan National University Yangsan Hospital, Pusan National University School of Medicine, Yangsan, Korea.
- KMID: 2468165
- DOI: http://doi.org/10.4285/jkstn.2019.33.4.146
Abstract
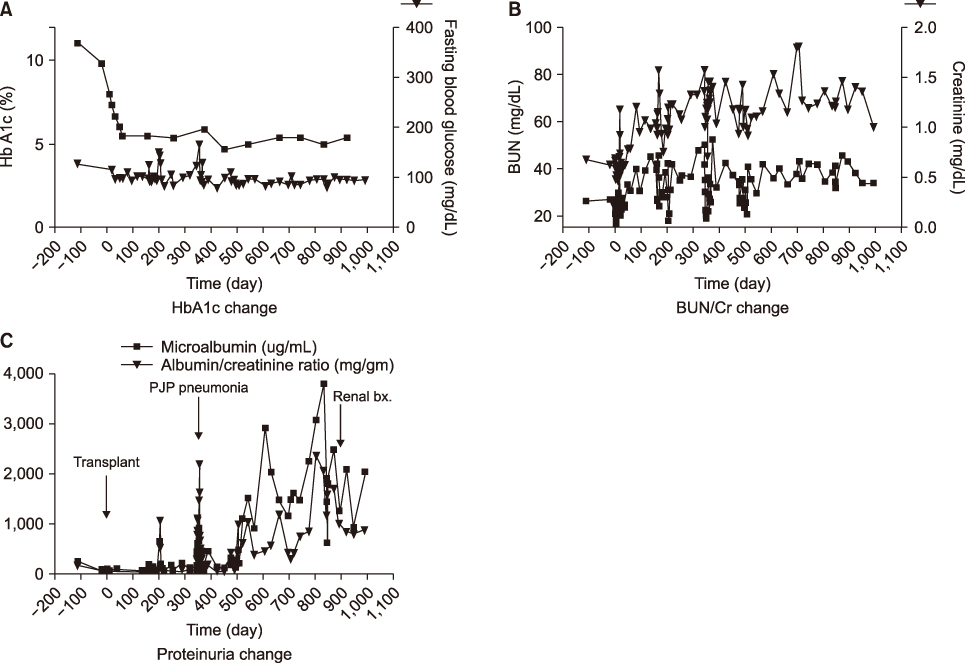
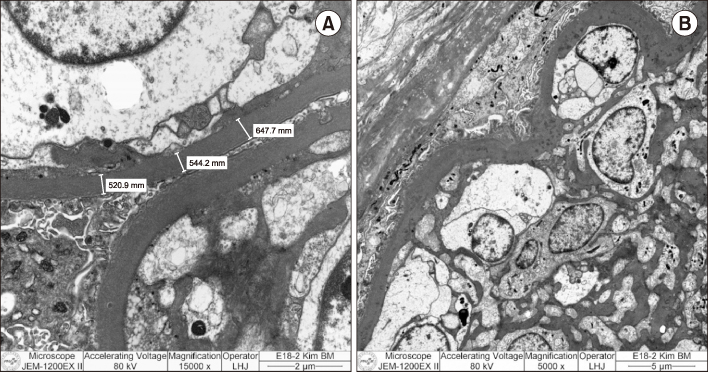
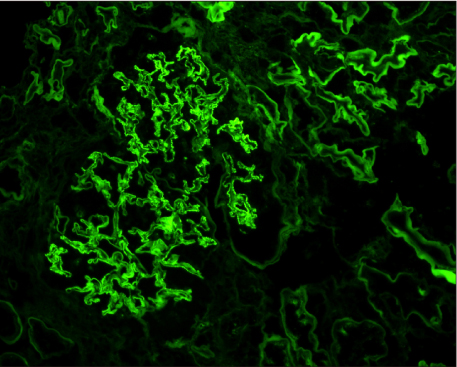
- Pancreas transplantation is the only method that can nearly cure insulin-dependent diabetes mellitus. However, the effect of pancreas transplantation on patients with diabetic nephropathy has recently been considered controversial. In this report, we present a case of abrupt aggravation of proteinuria after successful pancreas transplantation alone without evidence of calcineurin inhibitor (CNI) toxicity. A 22-year-old female patient with type I diabetes mellitus underwent pancreas transplantation alone. The patient already had retinopathy and mild proteinuria, which in this case, may mean diabetic nephropathy. Her glucose levels were managed within the normal range after successful pancreas transplantation. However, the amount of proteinuria fluctuated. Kidney needle biopsy was performed owing to severe elevation of proteinuria, 2 years after the transplantation. Electron microscopy revealed diabetic glomerulosclerosis without evidence of CNI toxicity. This case indicates that diabetic nephropathy can be aggravated after pancreas transplantation, despite well-managed glucose levels and absence of CNI toxicity.
MeSH Terms
Figure
Reference
-
1. Dean PG, Kukla A, Stegall MD, Kudva YC. Pancreas transplantation. BMJ. 2017; 357:j1321.
Article2. Wisel SA, Braun HJ, Stock PG. Current outcomes in islet versus solid organ pancreas transplant for β-cell replacement in type 1 diabetes. Curr Opin Organ Transplant. 2016; 21:399–404.
Article3. Bekiari E, Kitsios K, Thabit H, Tauschmann M, Athanasiadou E, Karagiannis T, et al. Artificial pancreas treatment for outpatients with type 1 diabetes: systematic review and meta-analysis. BMJ. 2018; 361:k1310.
Article4. Redfield RR, Rickels MR, Naji A, Odorico JS. Pancreas transplantation in the modern era. Gastroenterol Clin North Am. 2016; 45:145–166.
Article5. Shin S, Jung CH, Choi JY, Kwon HW, Jung JH, Kim YH, et al. Long-term effects of pancreas transplant alone on nephropathy in type 1 diabetic patients with optimal renal function. PLoS One. 2018; 13:e0191421.
Article6. Ruospo M, Saglimbene VM, Palmer SC, De Cosmo S, Pacilli A, Lamacchia O, et al. Glucose targets for preventing diabetic kidney disease and its progression. Cochrane Database Syst Rev. 2017; 6:CD010137.
Article7. Ryu JH, Lee TB, Park YM, Yang KH, Chu CW, Lee JH, et al. Pancreas transplant with duodeno-duodenostomy and caval drainage using a diamond patch graft: a single-center experience. Ann Transplant. 2017; 22:24–34.
Article8. Fioretto P, Steffes MW, Sutherland DE, Goetz FC, Mauer M. Reversal of lesions of diabetic nephropathy after pancreas transplantation. N Engl J Med. 1998; 339:69–75.
Article9. Boggi U, Vistoli F, Amorese G, Giannarelli R, Coppelli A, Mariotti R, et al. Results of pancreas transplantation alone with special attention to native kidney function and proteinuria in type 1 diabetes patients. Rev Diabet Stud. 2011; 8:259–267.
Article10. Fioretto P, Barzon I, Mauer M. Is diabetic nephropathy reversible? Diabetes Res Clin Pract. 2014; 104:323–328.
Article11. Mauer M, Fioretto P. Pancreas transplantation and reversal of diabetic nephropathy lesions. Med Clin North Am. 2013; 97:109–114.
Article12. Singh SK, Kim SJ, Smail N, Schiff J, Paraskevas S, Cantarovich M. Outcomes of recipients with pancreas transplant alone who develop end-stage renal disease. Am J Transplant. 2016; 16:535–540.
Article13. Maahs DM. Early detection of kidney disease in type 1 diabetes: what do we really know? Diabetes Technol Ther. 2012; 14:541–544.
Article14. Bjornstad P, Cherney D, Maahs DM. Early diabetic nephropathy in type 1 diabetes: new insights. Curr Opin Endocrinol Diabetes Obes. 2014; 21:279–286.15. Lin YC, Chang YH, Yang SY, Wu KD, Chu TS. Update of pathophysiology and management of diabetic kidney disease. J Formos Med Assoc. 2018; 117:662–675.
Article16. Kameda Y, Babazono T, Uchigata Y, Kitano S. Renal function after intravitreal administration of vascular endothelial growth factor inhibitors in patients with diabetes and chronic kidney disease. J Diabetes Investig. 2018; 9:937–939.
Article17. Lafayette RA, McCall B, Li N, Chu L, Werner P, Das A, et al. Incidence and relevance of proteinuria in bevacizumab-treated patients: pooled analysis from randomized controlled trials. Am J Nephrol. 2014; 40:75–83.
Article18. Hanna RM, Lopez E, Wilson J, Barathan S, Cohen AH. Minimal change disease onset observed after bevacizumab administration. Clin Kidney J. 2016; 9:239–244.
Article19. Pérez-Valdivia MA, López-Mendoza M, Toro-Prieto FJ, Cabello-Chaves V, Toro-Ramos M, Martín-Herrera MC, et al. Relapse of minimal change disease nephrotic syndrome after administering intravitreal bevacizumab. Nefrologia. 2014; 34:421–422.