Nat Prod Sci.
2019 Dec;25(4):354-357. 10.20307/nps.2019.25.4.354.
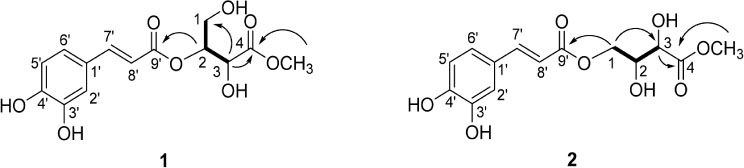
Two New Caffeoyl Threonate Esters from the Leaves of Toxicodendron vernicifluum
- Affiliations
-
- 1College of Pharmacy, Chungbuk National University, Cheongju, Korea. mklee@chungbuk.ac.kr
- 2Department of Aqualife Medicine, Chonnam National University, Yeosu, Korea.
- KMID: 2468070
- DOI: http://doi.org/10.20307/nps.2019.25.4.354
Abstract
- Toxicodendron vernicifluum, also called as Rhus verniciflua is a deciduous tree belonging to Anacardiaceae family. Two new caffeoyl threonate esters, rhuseols A (1) and B (2), together with 5-O-(E)-caffeoylquinic acid methyl ester (3) were isolated from the leaves of T. vernicifluum. The structures of isolated compounds were established by using 1D and 2D NMR in combination with HR-ESI-MS. Compounds 1 - 3 showed DPPH radical scavenging effects with ICâ‚…â‚€ values of 47.9, 107.8 and 15.4 µM, respectively. Taken together, these compounds might contribute to the antioxidant properties of the leaves of T. vernicifluum, which will be useful for various oxidative stress mediated diseases.
MeSH Terms
Figure
Reference
-
1. Kim JS, Kwon YS, Chun WJ, Kim TY, Sun J, Yu CY, Kim MJ. Food Chem. 2010; 120:539–543.2. Lee SO, Kim SJ, Kim JS, Ji H, Lee EO, Lee HJ. BMC Complement Altern Med. 2018; 18:242.3. Park KY, Jung GO, Lee KT, Choi J, Choi MY, Kim GT, Jung HJ, Park HJ. J Ethnopharmacol. 2004; 90:73–79.4. Lee JH, Lee HJ, Lee HJ, Choi WC, Yoon SW, Ko SG, Ahn KS, Choi SH, Ahn KS, Lieske JC, Kim SH. Phytomedicine. 2009; 16:188–197.5. Agu KC, Okolie PN. Food Sci Nutr. 2017; 5:1029–1036.
Article6. Zeng H, Su S, Xiang X, Sha X, Zhu Z, Wang Y, Guo S, Yan H, Qian D, Duan J. Molecules. 2017; 22:E771.7. Wei JN, Liu ZH, Zhao YP, Zhao LL, Xue TK, Lan QK. Food Chem. 2019; 286:260–267.8. Pompermaier L, Marzocco S, Adesso S, Monizi M, Schwaiger S, Neinhuis C, Stuppner H, Lautenschläger T. J Ethnopharmacol. 2018; 216:26–36.9. Jang JY, Shin H, Lim JW, Ahn JH, Jo YH, Lee KY, Hwang BY, Jung SJ, Kang SY, Lee MK. PLoS One. 2018; 13:e0200257.10. Kim KH, Moon E, Choi SU, Kim SY, Lee KR. Phytochemistry. 2013; 92:113–121.11. Chen H, Wang C, Ye J, Zhou H, Yuan J. Nat Prod Res. 2014; 28:496–499.12. Kang SY. J Fish Pathol. 2005; 18:227–237.13. Kuczkowiak U, Perereit F, Nahrstedt A. Sci Pharm. 2014; 82:835–846.14. Silva DB, Okano LT, Lopes NP, de Oliveira DCR. Phytochemisty. 2013; 96:418–422.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Lipid Composition of Serum HDL and Fatty Acid Composition of Serum Cholesteryl Esters in Newborn
- Protoporphyrin IX Synthesis Induced by 5-aminolevulinic Acid and Its Esters in Normal Mouse Skin
- Experimental Study on Latent Sensitivity to Rhus Trees
- Anti-nociceptive and Anti-inflammatory Properties of Ilex latifolia and its Active Component, 3,5-Di-caffeoyl Quinic Acid Methyl Ester
- Phorbol Esters Attenuate The Action of Isoproterenol on Vascular Smooth Muscle