Korean J Radiol.
2020 Jan;21(1):42-57. 10.3348/kjr.2019.0354.
Neuroimaging in Randomized, Multi-Center Clinical Trials of Endovascular Treatment for Acute Ischemic Stroke: A Systematic Review
- Affiliations
-
- 1Department of Radiology and Research Institute of Radiology, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea. dynamics79@gmail.com
- 2Department of Radiology, Korea University Anam Hospital, Korea University College of Medicine, Seoul, Korea.
- 3Bioimaging Center, Biomedical Research Center, Asan Institute for Life Sciences, Asan Medical Center, Seoul, Korea.
- 4Clinical Research Division, National Institute of Food and Drug Safety Evaluation, Ministry of Food and Drug Safety, Cheongju, Korea.
- 5Asan Image Metrics, Clinical Trial Center, Asan Institute for Life Sciences, Asan Medical Center, Seoul, Korea.
- KMID: 2467041
- DOI: http://doi.org/10.3348/kjr.2019.0354
Abstract
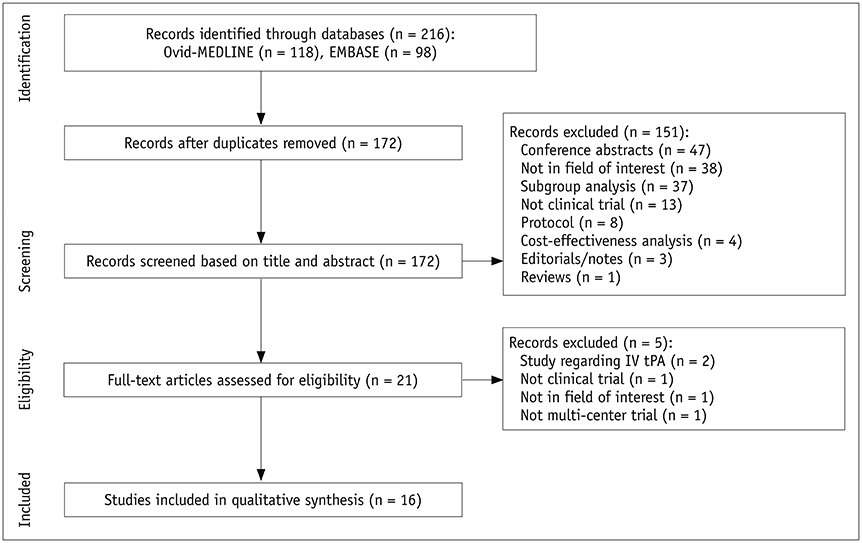
- Appropriate use and analysis of neuroimaging techniques is an inevitable aspect of clinical trials for patients with acute ischemic stroke. Neuroimaging examinations were recently used to define the core eligibility criteria and outcomes in acute ischemic stroke research. Recent clinical trials for endovascular treatment in acute ischemic stroke have also demonstrated the efficacy or safety of endovascular treatment using various imaging modalities as well as clinical indices. Furthermore, independent imaging reviews and imaging core laboratory assessments are essential to manage and analyze imaging data in order to enhance the reliability of the outcomes. Therefore, we systematically reviewed the use of neuroimaging in recent randomized clinical trials for endovascular treatment of acute ischemic stroke in order to provide a thorough summary, which would serve as a resource guiding the use of appropriate imaging protocols and analyses in future clinical trials for acute ischemic stroke. This review will help researchers select appropriate imaging biomarkers among the various imaging protocols available and apply the selected type of imaging examination for each study in accordance with the academic purpose.
Keyword
Figure
Cited by 1 articles
-
Proposed Protocols for Artificial Intelligence Imaging Database in Acute Stroke Imaging
Minjae Kim, Seung Chai Jung, Soo Chin Kim, Bum Joon Kim, Woo-Keun Seo, Byungjun Kim
Neurointervention. 2023;18(3):149-158. doi: 10.5469/neuroint.2023.00339.
Reference
-
1. Lee JS, Demchuk AM. Choosing a hyperacute stroke imaging protocol for proper patient selection and time efficient endovascular treatment: lessons from recent trials. J Stroke. 2015; 17:221–228.
Article2. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. American Heart Association Stroke Council. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018; 49:e46–e110.
Article3. Hacke W, Kaste M, Fieschi C, Toni D, Lesaffre E, von Kummer R, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA. 1995; 274:1017–1025.
Article4. National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995; 333:1581–1587.5. Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. DAWN Trial Investigators. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018; 378:11–21.6. Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, et al. DEFUSE 3 Investigators. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018; 378:708–718.
Article7. Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al. SWIFT PRIME Investigators. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015; 372:2285–2295.
Article8. Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al. REVASCAT Trial Investigators. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015; 372:2296–2306.
Article9. Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J. ESCAPE Trial Investigators. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015; 372:1019–1030.10. Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. EXTEND-IA Investigators. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015; 372:1009–1018.11. Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. MR CLEAN Investigators. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015; 372:11–20.12. Muir KW, Ford GA, Messow CM, Ford I, Murray A, Clifton A, et al. PISTE Investigators. Endovascular therapy for acute ischaemic stroke: the Pragmatic Ischaemic Stroke Thrombectomy Evaluation (PISTE) randomised, controlled trial. J Neurol Neurosurg Psychiatry. 2017; 88:38–44.
Article13. Lapergue B, Blanc R, Gory B, Labreuche J, Duhamel A, Marnat G, et al. ASTER Trial Investigators. Effect of endovascular contact aspiration vs stent retriever on revascularization in patients with acute ischemic stroke and large vessel occlusion: the ASTER randomized clinical trial. JAMA. 2017; 318:443–452.
Article14. Mocco J, Zaidat OO, von Kummer R, Yoo AJ, Gupta R, Lopes D, et al. THERAPY Trial Investigators. Aspiration thrombectomy after intravenous alteplase versus intravenous alteplase alone. Stroke. 2016; 47:2331–2338.
Article15. Bracard S, Ducrocq X, Mas JL, Soudant M, Oppenheim C, Moulin T, et al. THRACE investigators. Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurol. 2016; 15:1138–1147.
Article16. Kidwell CS, Jahan R, Gornbein J, Alger JR, Nenov V, Ajani Z, et al. MR RESCUE Investigators. A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med. 2013; 368:914–923.
Article17. Ciccone A, Valvassori L, Nichelatti M, Sgoifo A, Ponzio M, Sterzi R, et al. SYNTHESIS Expansion Investigators. Endovascular treatment for acute ischemic stroke. N Engl J Med. 2013; 368:904–913.
Article18. Broderick JP, Palesch YY, Demchuk AM, Yeatts SD, Khatri P, Hill MD, et al. Interventional Management of Stroke (IMS) III Investigators. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med. 2013; 368:893–903.
Article19. Saver JL, Jahan R, Levy EI, Jovin TG, Baxter B, Nogueira RG, et al. SWIFT Trialists. Solitaire flow restoration device versus the Merci retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel-group, non-inferiority trial. Lancet. 2012; 380:1241–1249.
Article20. Nogueira RG, Lutsep HL, Gupta R, Jovin TG, Albers GW, Walker GA, et al. TREVO 2 Trialists. Trevo versus Merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke (TREVO 2): a randomised trial. Lancet. 2012; 380:1231–1240.
Article21. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009; 151:W65–W94.
Article22. Campbell BCV, Mitchell PJ, Churilov L, Yassi N, Kleinig TJ, Dowling RJ, et al. EXTEND-IA TNK Investigators. Tenecteplase versus alteplase before thrombectomy for ischemic stroke. N Engl J Med. 2018; 378:1573–1582.23. Logallo N, Novotny V, Assmus J, Kvistad CE, Alteheld L, Rønning OM, et al. Tenecteplase versus alteplase for management of acute ischaemic stroke (NOR-TEST): a phase 3, randomised, open-label, blinded endpoint trial. Lancet Neurol. 2017; 16:781–788.
Article24. Wouters A, Lemmens R, Christensen S, Wilms G, Dupont P, Mlynash M, et al. AXIS 2 and DEFUSE 2 study investigators. Magnetic resonance imaging-based endovascular versus medical stroke treatment for symptom onset up to 12 h. Int J Stroke. 2016; 11:127–133.25. Schönenberger S, Uhlmann L, Hacke W, Schieber S, Mundiyanapurath S, Purrucker JC, et al. Effect of conscious sedation vs general anesthesia on early neurological improvement among patients with ischemic stroke undergoing endovascular thrombectomy: a randomized clinical trial. JAMA. 2016; 316:1986–1996.26. Khoury NN, Darsaut TE, Ghostine J, Deschaintre Y, Daneault N, Durocher A, et al. EASI trial collaborators. Endovascular thrombectomy and medical therapy versus medical therapy alone in acute stroke: a randomized care trial. J Neuroradiol. 2017; 44:198–202.
Article27. Kosior JC, Idris S, Dowlatshahi D, Alzawahmah M, Eesa M, Sharma P, et al. PREDICT/Sunnybrook CTA ICH study investigators. Quantomo: validation of a computer-assisted methodology for the volumetric analysis of intracerebral haemorrhage. Int J Stroke. 2011; 6:302–305.
Article28. Boers AM, Marquering HA, Jochem JJ, Besselink NJ, Berkhemer OA, van der Lugt A, et al. MR CLEAN investigators. Automated cerebral infarct volume measurement in follow-up noncontrast CT scans of patients with acute ischemic stroke. AJNR Am J Neuroradiol. 2013; 34:1522–1527.
Article29. Wardlaw JM, von Kummer R, Carpenter T, Parsons M, Lindley RI, Cohen G, et al. Protocol for the perfusion and angiography imaging sub-study of the Third International Stroke Trial (IST-3) of alteplase treatment within six-hours of acute ischemic stroke. Int J Stroke. 2015; 10:956–968.
Article30. Wahlgren N, Ahmed N, Dávalos A, Ford GA, Grond M, Hacke W, et al. SITS-MOST investigators. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet. 2007; 369:275–282.
Article31. Wintermark M, Albers GW, Alexandrov AV, Alger JR, Bammer R, Baron JC, et al. Acute stroke imaging research roadmap. Stroke. 2008; 39:1621–1628.
Article32. Saver JL, Jahan R, Levy EI, Jovin TG, Baxter B, Nogueira R, et al. SWIFT Trialists. SOLITAIRE™ with the intention for thrombectomy (SWIFT) trial: design of a randomized, controlled, multicenter study comparing the SOLITAIRE™ flow restoration device and the MERCI retriever in acute ischaemic stroke. Int J Stroke. 2014; 9:658–668.
Article33. Coutts SB, Demchuk AM, Barber PA, Hu WY, Simon JE, Buchan AM, et al. VISION Study Group. Interobserver variation of ASPECTS in real time. Stroke. 2004; 35:e103–e105.
Article34. Gupta AC, Schaefer PW, Chaudhry ZA, Leslie-Mazwi TM, Chandra RV, González RG, et al. Interobserver reliability of baseline noncontrast CT Alberta Stroke Program early CT score for intra-arterial stroke treatment selection. AJNR Am J Neuroradiol. 2012; 33:1046–1049.
Article35. Zaidat OO, Yoo AJ, Khatri P, Tomsick TA, von Kummer R, Saver JL, et al. Cerebral Angiographic Revascularization Grading (CARG) Collaborators. STIR Revascularization working group. STIR Thrombolysis in Cerebral Infarction (TICI) Task Force. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke. 2013; 44:2650–2663.36. Zaidat OO, Lazzaro MA, Liebeskind DS, Janjua N, Wechsler L, Nogueira RG, et al. Revascularization grading in endovascular acute ischemic stroke therapy. Neurology. 2012; 79:13 Suppl 1. S110–S116.
Article37. Carrillo MC, Rowe CC, Szoeke C, Masters CL, Ames D, O'Meara T, et al. NIA/Alzheimer Association and International Working Group. Research and standardization in Alzheimer's trials: reaching international consensus. Alzheimers Dement. 2013; 9:160–168.
Article38. Weiner MW, Veitch DP, Aisen PS, Beckett LA, Cairns NJ, Green RC, et al. Alzheimer's Disease Neuroimaging Initiative. Recent publications from the Alzheimer's Disease Neuroimaging Initiative: reviewing progress toward improved AD clinical trials. Alzheimers Dement. 2017; 13:e1–e85.
Article39. deSouza NM, Winfield JM, Waterton JC, Weller A, Papoutsaki MV, Doran SJ, et al. Implementing diffusion-weighted MRI for body imaging in prospective multicentre trials: current considerations and future perspectives. Eur Radiol. 2018; 28:1118–1131.
Article40. Shukla-Dave A, Obuchowski NA, Chenevert TL, Jambawalikar S, Schwartz LH, Malyarenko D, et al. Quantitative imaging biomarkers alliance (QIBA) recommendations for improved precision of DWI and DCE-MRI derived biomarkers in multicenter oncology trials. J Magn Reson Imaging. 2019; 49:e101–e121.41. Packer RA, Rossmeisl JH, Kent MS, Griffin JF 4th, Mazcko C, LeBlanc AK. Consensus recommendations on standardized magnetic resonance imaging protocols for multicenter canine brain tumor clinical trials. Vet Radiol Ultrasound. 2018; 59:261–271.
Article42. Ellingson BM, Bendszus M, Boxerman J, Barboriak D, Erickson BJ, Smits M, et al. Jumpstarting Brain Tumor Drug Development Coalition Imaging Standardization Steering Committee. Consensus recommendations for a standardized Brain Tumor Imaging Protocol in clinical trials. Neuro Oncol. 2015; 17:1188–1198.43. Warach SJ, Luby M, Albers GW, Bammer R, Bivard A, Campbell BC, et al. Stroke Imaging Research (STIR) and VISTA-Imaging Investigators. Acute stroke imaging research roadmap III imaging selection and outcomes in acute stroke reperfusion clinical trials: consensus recommendations and further research priorities. Stroke. 2016; 47:1389–1398.44. Wintermark M, Albers GW, Broderick JP, Demchuk AM, Fiebach JB, Fiehler J, et al. Stroke Imaging Research (STIR) and Virtual International Stroke Trials Archive (VISTA)-Imaging Investigators. Acute stroke imaging research roadmap II. Stroke. 2013; 44:2628–2639.45. Heit JJ, Wintermark M. Perfusion computed tomography for the evaluation of acute ischemic stroke: strengths and pitfalls. Stroke. 2016; 47:1153–1158.46. Lin L, Bivard A, Parsons MW. Perfusion patterns of ischemic stroke on computed tomography perfusion. J Stroke. 2013; 15:164–173.
Article47. Vilela P, Rowley HA. Brain ischemia: CT and MRI techniques in acute ischemic stroke. Eur J Radiol. 2017; 96:162–172.
Article48. Wintermark M, Reichhart M, Thiran JP, Maeder P, Chalaron M, Schnyder P, et al. Prognostic accuracy of cerebral blood flow measurement by perfusion computed tomography, at the time of emergency room admission, in acute stroke patients. Ann Neurol. 2002; 51:417–432.
Article49. Vert C, Parra-Fariñas C, Rovira À. MR imaging in hyperacute ischemic stroke. Eur J Radiol. 2017; 96:125–132.
Article50. Eliasziw M, Paddock-Eliasziw L. Comparison of MRI and CT for detection of acute intracerebral hemorrhage. JAMA. 2005; 293:550. author reply 550–551.
Article51. Arnould MC, Grandin CB, Peeters A, Cosnard G, Duprez TP. Comparison of CT and three MR sequences for detecting and categorizing early (48 hours) hemorrhagic transformation in hyperacute ischemic stroke. AJNR Am J Neuroradiol. 2004; 25:939–944.52. Neeb L, Villringer K, Galinovic I, Grosse-Dresselhaus F, Ganeshan R, Gierhake D, et al. Adapting the computed tomography criteria of hemorrhagic transformation to stroke magnetic resonance imaging. Cerebrovasc Dis Extra. 2013; 3:103–110.
Article53. Caplan LR. Vertebrobasilar ischemia and hemorrhage: clinical findings, diagnosis and management of posterior circulation disease. 2nd ed. Cambridge: Cambridge University Press;2015.54. Nouh A, Remke J, Ruland S. Ischemic posterior circulation stroke: a review of anatomy, clinical presentations, diagnosis, and current management. Front Neurol. 2014; 5:30.
Article55. Wintermark M, Rowley HA, Lev MH. Acute stroke triage to intravenous thrombolysis and other therapies with advanced CT or MR imaging: pro CT. Radiology. 2009; 251:619–626.
Article56. Kim JS, Caplan LR, Wong KS. Intracranial atherosclerosis: pathophysiology, diagnosis and treatment. Basel: Karger;2016.57. Yang JJ, Hill MD, Morrish WF, Hudon ME, Barber PA, Demchuk AM, et al. Comparison of pre- and postcontrast 3D time-of-flight MR angiography for the evaluation of distal intracranial branch occlusions in acute ischemic stroke. AJNR Am J Neuroradiol. 2002; 23:557–567.58. Shah S, Luby M, Poole K, Morella T, Keller E, Benson RT, et al. Screening with MRI for accurate and rapid stroke treatment: SMART. Neurology. 2015; 84:2438–2444.
Article59. Nael K, Khan R, Choudhary G, Meshksar A, Villablanca P, Tay J, et al. Six-minute magnetic resonance imaging protocol for evaluation of acute ischemic stroke: pushing the boundaries. Stroke. 2014; 45:1985–1991.60. Chung MS, Lee JY, Jung SC, Baek S, Shim WH, Park JE, et al. Reliability of fast magnetic resonance imaging for acute ischemic stroke patients using a 1.5-T scanner. Eur Radiol. 2019; 29:2641–2650.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Endovascular Treatment of Acute Ischemic Stroke
- Neuroimaging of Acute Ischemic Stroke: Multimodal Imaging Approach for Acute Endovascular Therapy
- Reperfusion therapy in acute ischemic stroke
- Endovascular Thrombectomy for Acute Ischemic Stroke : Current Concept in Management
- Choosing a Hyperacute Stroke Imaging Protocol for Proper Patient Selection and Time Efficient Endovascular Treatment: Lessons from Recent Trials