J Stroke.
2023 Jan;25(1):55-71. 10.5853/jos.2022.03286.
Neuroimaging of Acute Ischemic Stroke: Multimodal Imaging Approach for Acute Endovascular Therapy
- Affiliations
-
- 1Department of Radiology, Boston Medical Center, Boston, MA, USA
- 2Cooper Neurological Institute, Cooper University Hospital, Camden, NJ, USA
- 3Department of Neurology, Ajou University Hospital, Ajou University School of Medicine, Suwon, Korea
- 4Department of Neurology, Brown University, Providence, RI, USA
- 5Department of Neurology, The 903rd Hospital of The Chinese People’s Liberation Army, Hangzhou, China
- 6Department of Interventional Neuroradiology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
- 7Department of Medicine and Neurology, Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria, Australia
- 8Department of Neurology, Boston Medical Center, Boston, MA, USA
- KMID: 2539058
- DOI: http://doi.org/10.5853/jos.2022.03286
Abstract
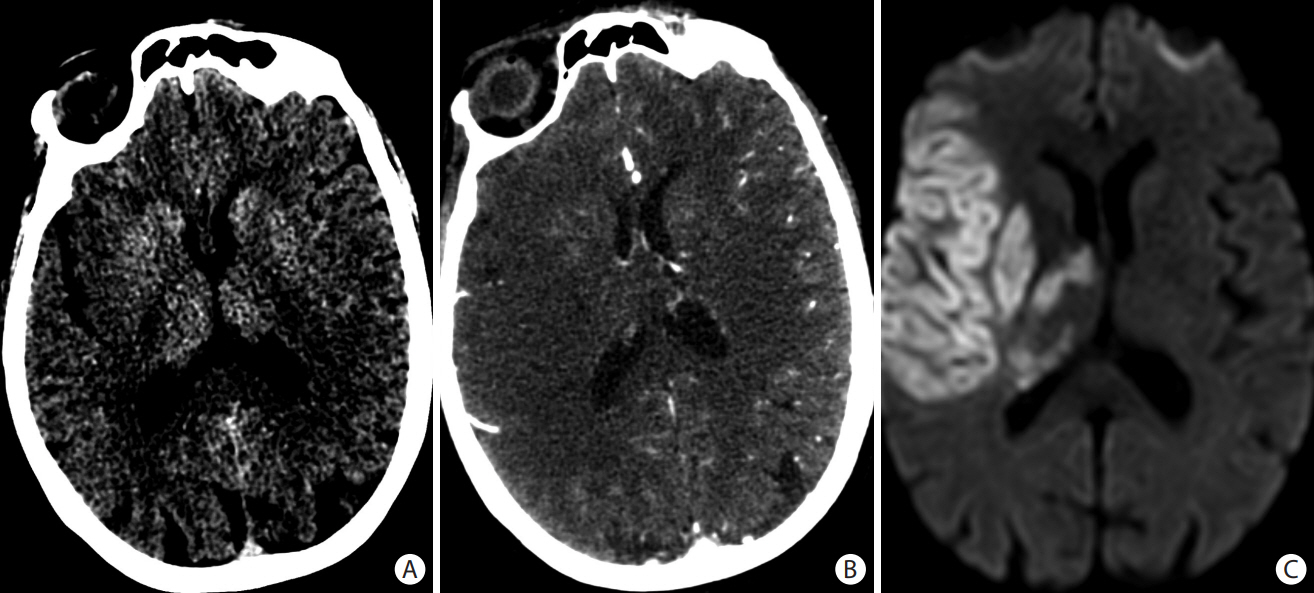
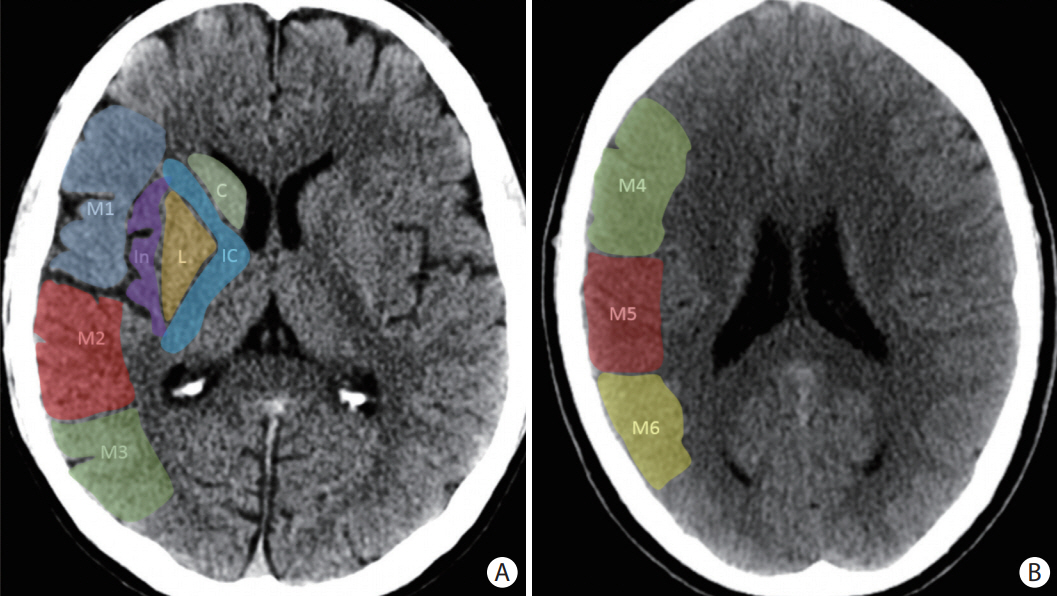
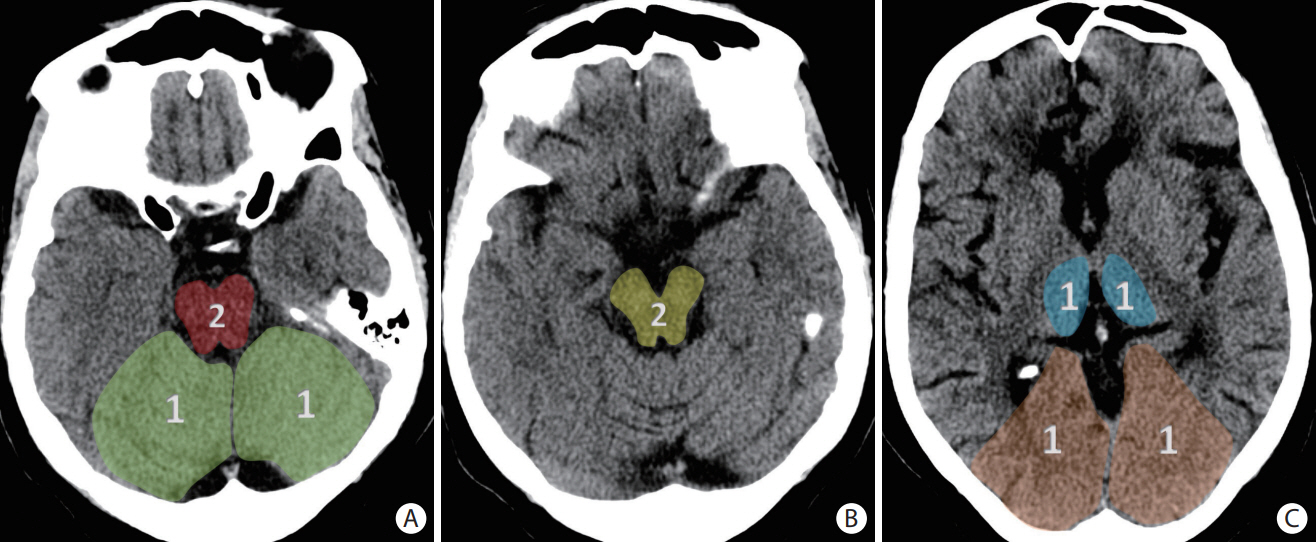
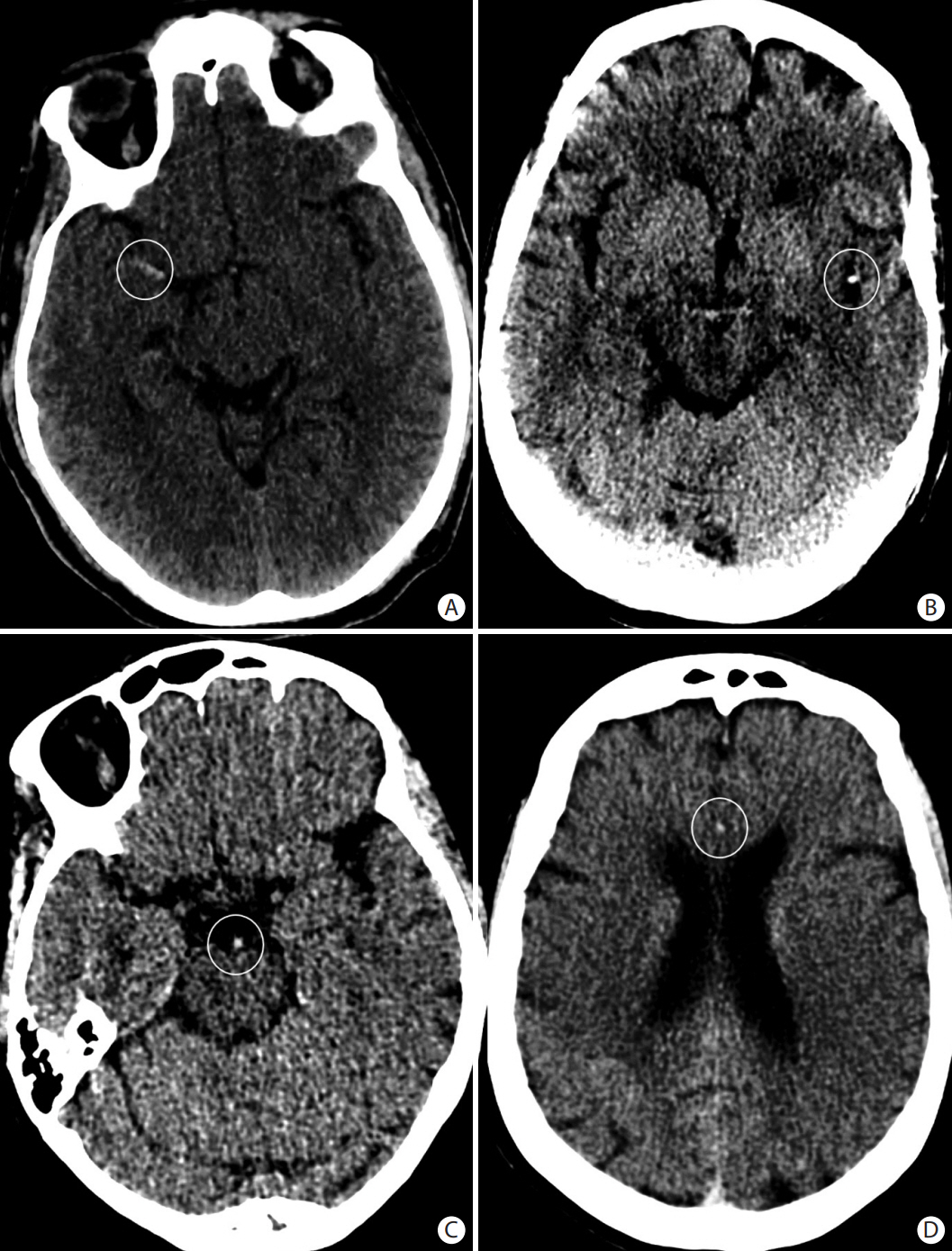
- Advances in acute ischemic stroke (AIS) treatment have been contingent on innovations in neuroimaging. Neuroimaging plays a pivotal role in the diagnosis and prognosis of ischemic stroke and large vessel occlusion, enabling triage decisions in the emergent care of the stroke patient. Current imaging protocols for acute stroke are dependent on the available resources and clinicians’ preferences and experiences. In addition, differential application of neuroimaging in medical decision-making, and the rapidly growing evidence to support varying paradigms have outpaced guideline-based recommendations for selecting patients to receive intravenous or endovascular treatment. In this review, we aimed to discuss the various imaging modalities and approaches used in the diagnosis and treatment of AIS.
Keyword
Figure
Reference
-
References
1. Romero JR, Pikula A, Nguyen TN, Nien YL, Norbash A, Babikian VL. Cerebral collateral circulation in carotid artery disease. Curr Cardiol Rev. 2009; 5:279–288.2. Meinel TR, Kaesmacher J, Mosimann PJ, Seiffge D, Jung S, Mordasini P, et al. Association of initial imaging modality and futile recanalization after thrombectomy. Neurology. 2020; 95:e2331–e2342.3. Linfante I, Starosciak AK, Walker GR, Dabus G, Castonguay AC, Gupta R, et al. Predictors of poor outcome despite recanalization: a multiple regression analysis of the NASA registry. J Neurointerv Surg. 2016; 8:224–229.4. Seker F, Qureshi MM, Möhlenbruch MA, Nogueira RG, Abdalkader M, Ribo M, et al. Reperfusion without functional independence in late presentation of stroke with large vessel occlusion. Stroke. 2022; 53:3594–3604.5. Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018; 378:708–718.6. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018; 49:e46–e110.7. Ko SB, Park HK, Kim BM, Heo JH, Rha JH, Kwon SU, et al. 2019 update of the Korean clinical practice guidelines of stroke for endovascular recanalization therapy in patients with acute ischemic stroke. J Stroke. 2019; 21:231–240.8. Liu L, Chen W, Zhou H, Duan W, Li S, Huo X, et al. Chinese Stroke Association guidelines for clinical management of cerebrovascular disorders: executive summary and 2019 update of clinical management of ischaemic cerebrovascular diseases. Stroke Vasc Neurol. 2020; 5:159–176.9. Casaubon LK, Boulanger JM, Blacquiere D, Boucher S, Brown K, Goddard T, et al. Canadian stroke best practice recommendations: hyperacute stroke care guidelines, update 2015. Int J Stroke. 2015; 10:924–940.10. Turc G, Bhogal P, Fischer U, Khatri P, Lobotesis K, Mazighi M, et al. European Stroke Organisation (ESO) - European Society for Minimally Invasive Neurological Therapy (ESMINT) guidelines on mechanical thrombectomy in Acute Ischaemic StrokeEndorsed by Stroke Alliance for Europe (SAFE). Eur Stroke J. 2019; 4:6–12.11. Nguyen TN, Castonguay AC, Siegler JE, Nagel S, Lansberg MG, de Havenon A, et al. Mechanical thrombectomy in the late presentation of anterior circulation large vessel occlusion stroke: a guideline from the Society of Vascular and Interventional Neurology guidelines and Practice Standards Committee. Stroke Vasc Interv Neurol. 2023; 3:e000512.12. Nguyen TN, Klein P, Berberich A, Nagel S, Abdalkader M, Herning A, et al. Late window imaging selection for endovascular therapy of large vessel occlusion stroke: an international survey. Stroke Vasc Interv Neurol. 2023; 3:e000595.13. Nguyen TN, Abdalkader M, Nagel S, Qureshi MM, Ribo M, Caparros F, et al. Noncontrast computed tomography vs computed tomography perfusion or magnetic resonance imaging selection in late presentation of stroke with large-vessel occlusion. JAMA Neurol. 2022; 79:22–31.14. Sheth KN, Terry JB, Nogueira RG, Horev A, Nguyen TN, Fong AK, et al. Advanced modality imaging evaluation in acute ischemic stroke may lead to delayed endovascular reperfusion therapy without improvement in clinical outcomes. J Neurointerv Surg. 2013; 5 Suppl 1:i62–i65.15. Goyal M, Menon BK, Derdeyn CP. Perfusion imaging in acute ischemic stroke: let us improve the science before changing clinical practice. Radiology. 2013; 266:16–21.16. Gao J, Parsons MW, Kawano H, Levi CR, Evans TJ, Lin L, et al. Visibility of CT early ischemic change is significantly associated with time from stroke onset to baseline scan beyond the first 3 hours of stroke onset. J Stroke. 2017; 19:340–346.17. Demchuk AM, Hill MD, Barber PA, Silver B, Patel SC, Levine SR; NINDS rtPA Stroke Study Group. Importance of early ischemic computed tomography changes using ASPECTS in NINDS rtPA Stroke Study. Stroke. 2005; 36:2110–2115.18. Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. Lancet. 2000; 355:1670–1674.19. Yu W, Jiang WJ. A simple imaging guide for endovascular thrombectomy in acute ischemic stroke: from time window to perfusion mismatch and beyond. Front Neurol. 2019; 10:502.20. Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016; 387:1723–1731.21. Román LS, Menon BK, Blasco J, Hernández-Pérez M, Dávalos A, Majoie CBLM, et al. Imaging features and safety and efficacy of endovascular stroke treatment: a meta-analysis of individual patient-level data. Lancet Neurol. 2018; 17:895–904.22. Yoshimura S, Sakai N, Yamagami H, Uchida K, Beppu M, Toyoda K, et al. Endovascular therapy for acute stroke with a large ischemic region. N Engl J Med. 2002; 386:1303–1313.23. Schröder J, Cheng B, Ebinger M, Köhrmann M, Wu O, Kang DW, et al. Validity of acute stroke lesion volume estimation by diffusion-weighted imaging-Alberta stroke program early computed tomographic score depends on lesion location in 496 patients with middle cerebral artery stroke. Stroke. 2014; 45:3583–3588.24. Coutts SB, Lev MH, Eliasziw M, Roccatagliata L, Hill MD, Schwamm LH, et al. ASPECTS on CTA source images versus unenhanced CT: added value in predicting final infarct extent and clinical outcome. Stroke. 2004; 35:2472–2476.25. Puetz V, Khomenko A, Hill MD, Dzialowski I, Michel P, Weimar C, et al. Extent of hypoattenuation on CT angiography source images in basilar artery occlusion: prognostic value in the Basilar Artery International Cooperation Study. Stroke. 2001; 42:3454–3459.26. Puetz V, Sylaja PN, Coutts SB, Hill MD, Dzialowski I, Mueller P, et al. Extent of hypoattenuation on CT angiography source images predicts functional outcome in patients with basilar artery occlusion. Stroke. 2008; 39:2485–2490.27. Jovin TG, Li C, Wu L, Wu C, Chen J, Jiang C, et al. Trial of thrombectomy 6 to 24 hours after stroke due to basilar-artery occlusion. N Engl J Med. 2022; 387:1373–1384.28. Tao C, Nogueira RG, Zhu Y, Sun J, Han H, Yuan G, et al. Trial of endovascular treatment of acute basilar-artery occlusion. N Engl J Med. 2022; 387:1361–1372.29. Abdalkader M, Hu W. Endovascular therapy for stroke due to basilar artery occlusion: challenges and opportunities. J Neuroradiol. 2022; Dec. 14. [Epub]. https://doi.org/10.1016/j.neurad.2022.12.004.30. Mair G, Boyd EV, Chappell FM, von Kummer R, Lindley RI, Sandercock P, et al. Sensitivity and specificity of the hyperdense artery sign for arterial obstruction in acute ischemic stroke. Stroke. 2015; 46:102–107.31. Leys D, Pruvo JP, Godefroy O, Rondepierre P, Leclerc X. Prevalence and significance of hyperdense middle cerebral artery in acute stroke. Stroke. 1992; 23:317–324.32. Riedel CH, Zoubie J, Ulmer S, Gierthmuehlen J, Jansen O. Thin-slice reconstructions of nonenhanced CT images allow for detection of thrombus in acute stroke. Stroke. 2012; 43:2319–2323.33. Barber PA, Demchuk AM, Hudon ME, Pexman JH, Hill MD, Buchan AM. Hyperdense sylvian fissure MCA “dot” sign: a CT marker of acute ischemia. Stroke. 2001; 32:84–88.34. Goldmakher GV, Camargo EC, Furie KL, Singhal AB, Roccatagliata L, Halpern EF, et al. Hyperdense basilar artery sign on unenhanced CT predicts thrombus and outcome in acute posterior circulation stroke. Stroke. 2009; 40:134–139.35. Abdalkader M, Sahoo A, Shulman JG, Sader E, Takahashi C, Kaliaev A, et al. Acute occlusion of the fetal posterior cerebral artery: diagnosis and management paradigms. Neuroradiol J. 2021; Jun. 6. [Epub]. https://doi.org/10.1177/19714009211019383.36. Abdalkader M, Sahoo A, Dmytriw AA, Brinjikji W, Dabus G, Raz E, et al. Mechanical thrombectomy of the fetal posterior cerebral artery. Stroke Vasc Interv Neurol. 2021; 1:e000115.37. Brinjikji W, Duffy S, Burrows A, Hacke W, Liebeskind D, Majoie CBLM, et al. Correlation of imaging and histopathology of thrombi in acute ischemic stroke with etiology and outcome: a systematic review. J Neurointerv Surg. 2017; 9:529–534.38. Brouwer PA, Brinjikji W, De Meyer SF. Clot pathophysiology: why is it clinically important? Neuroimaging Clin N Am. 2018; 28:611–623.39. Nguyen TN, Castonguay AC, Nogueira RG, Haussen DC, English JD, Satti SR, et al. Effect of balloon guide catheter on clinical outcomes and reperfusion in Trevo thrombectomy. J Neurointerv Surg. 2019; 11:861–865.40. Broderick JP, Palesch YY, Demchuk AM, Yeatts SD, Khatri P, Hill MD, et al. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med. 2013; 368:893–903.41. Ciccone A, Valvassori L; SYNTHESIS Expansion Investigators. Endovascular treatment for acute ischemic stroke. N Engl J Med. 2013; 368:2433–2434.42. Qureshi AI, Abd-Allah F, Aleu A, Connors JJ, Hanel RA, Hassan AE, et al. Endovascular treatment for acute ischemic stroke patients: implications and interpretation of IMS III, MR RESCUE, and SYNTHESIS EXPANSION trials: a report from the working group of international congress of interventional neurology. J Vasc Interv Neurol. 2014; 7:56–75.43. Lee JS, Bang OY. Collateral status and outcomes after thrombectomy. Transl Stroke Res. 2022; Jun. 10. [Epub]. https://doi.org/10.1007/s12975-022-01046-z.44. Lee SU, Hong JM, Kim SY, Bang OY, Demchuk AM, Lee JS. Differentiating carotid terminus occlusions into two distinct populations based on Willisian collateral status. J Stroke. 2016; 18:179–186.45. Lee SJ, Hwang YH, Hong JM, Choi JW, Kang DH, Kim YW, et al. Predictors and prognoses of Willisian collateral failure during mechanical thrombectomy. Sci Rep. 2020; 10:20874.46. Rocha M, Jovin TG. Fast versus slow progressors of infarct growth in large vessel occlusion stroke: clinical and research implications. Stroke. 2017; 48:2621–2627.47. Berberich A, Finitsis S, Strambo D, Michel P, Herweh C, Meyer L, et al. Endovascular therapy versus no endovascular therapy in patients receiving best medical management for acute isolated occlusion of the posterior cerebral artery: a systematic review and meta-analysis. Eur J Neurol. 2022; 29:2664–2673.48. Herweh C, Abdalkader M, Nguyen TN, Puetz V, Schöne D, Kaiser D, et al. Mechanical thrombectomy in isolated occlusion of the proximal posterior cerebral artery. Front Neurol. 2021; 12:697348.49. Lee JS, Lee SJ, Hong JM, Alverne FJAM, Lima FO, Nogueira RG. Endovascular treatment of large vessel occlusion strokes due to intracranial atherosclerotic disease. J Stroke. 2022; 24:3–20.50. Diouf A, Fahed R, Gaha M, Chagnon M, Khoury N, Kotowski M, et al. Cervical internal carotid occlusion versus pseudo-occlusion at CT angiography in the context of acute stroke: an accuracy, interobserver, and intraobserver agreement study. Radiology. 2018; 286:1008–1015.51. Alverne FJAM, Lima FO, Rocha FA, Bandeira DA, Lucena AF, Silva HC, et al. Unfavorable vascular anatomy during endovascular treatment of stroke: challenges and bailout strategies. J Stroke. 2020; 22:185–202.52. Abdalkader M, Sahoo A, Lee J, Kiley N, Masoud HE, Norbash AM, et al. Balloon gliding technique: a novel use of balloon guiding catheters in accessing challenging circulations when treating acute ischemic stroke. Interv Neuroradiol. 2022; Mar. 14. [Epub]. https://doi.org/10.1177/15910199221082473.53. Cohnen M, Wittsack HJ, Assadi S, Muskalla K, Ringelstein A, Poll LW, et al. Radiation exposure of patients in comprehensive computed tomography of the head in acute stroke. AJNR Am J Neuroradiol. 2006; 27:1741–1745.54. Kaiser DPO, Abdalkader M, Berberich A, Sporns PB, Nguyen TN. Acute shortage of iodinated contrast media: implications and guidance for neurovascular imaging and intervention. Neuroradiology. 2002; 64:1715–1718.55. Kufner A, Erdur H, Endres M, Nolte CH, Scheel M, Schlemm L. Association between thrombus perviousness assessed on computed tomography and stroke cause. Stroke. 2020; 51:3613–3622.56. Kappelhof M, Tolhuisen ML, Treurniet KM, Dutra BG, Alves H, Zhang G, et al. Endovascular treatment effect diminishes with increasing thrombus perviousness: pooled data from 7 trials on acute ischemic stroke. Stroke. 2021; 52:3633–3641.57. Borst J, Berkhemer OA, Santos EMM, Yoo AJ, den Blanken M, Roos YBWEM, et al. Value of thrombus CT characteristics in patients with acute ischemic stroke. AJNR Am J Neuroradiol. 2017; 38:1758–1764.58. Wiegers EJA, Mulder MJHL, Jansen IGH, Venema E, Compagne KCJ, Berkhemer OA, et al. Clinical and imaging determinants of collateral status in patients with acute ischemic stroke in MR CLEAN trial and registry. Stroke. 2020; 51:1493–1502.59. Menon BK, d’Esterre CD, Qazi EM, Almekhlafi M, Hahn L, Demchuk AM, et al. Multiphase CT angiography: a new tool for the imaging triage of patients with acute ischemic stroke. Radiology. 2015; 275:510–520.60. Liebeskind DS. Collateral circulation. Stroke. 2003; 34:2279–2284.61. Bang OY, Goyal M, Liebeskind DS. Collateral circulation in ischemic stroke: assessment tools and therapeutic strategies. Stroke. 2015; 46:3302–3309.62. Wang YJ, Wang JQ, Qiu J, Li W, Sun XH, Zhao YG, et al. Association between collaterals, cerebral circulation time and outcome after thrombectomy of stroke. Ann Clin Transl Neurol. 2022; Dec. 16. [Epub]. https://doi.org/10.1002/acn3.51718.63. Marks MP, Lansberg MG, Mlynash M, Olivot JM, Straka M, Kemp S, et al. Effect of collateral blood flow on patients undergoing endovascular therapy for acute ischemic stroke. Stroke. 2014; 45:1035–1039.64. Vagal A, Aviv R, Sucharew H, Reddy M, Hou Q, Michel P, et al. Collateral clock is more important than time clock for tissue fate: a natural history study of acute ischemic strokes. Stroke. 2018; 49:2102–2107.65. Souza LC, Yoo AJ, Chaudhry ZA, Payabvash S, Kemmling A, Schaefer PW, et al. Malignant CTA collateral profile is highly specific for large admission DWI infarct core and poor outcome in acute stroke. AJNR Am J Neuroradiol. 2012; 33:1331–1336.66. Maas MB, Lev MH, Ay H, Singhal AB, Greer DM, Smith WS, et al. Collateral vessels on CT angiography predict outcome in acute ischemic stroke. Stroke. 2009; 40:3001–3005.67. Venema E, Mulder MJHL, Roozenbeek B, Broderick JP, Yeatts SD, Khatri P, et al. Selection of patients for intra-arterial treatment for acute ischaemic stroke: development and validation of a clinical decision tool in two randomised trials. BMJ. 2017; 357:j1710.68. d’Esterre CD, Trivedi A, Pordeli P, Boesen M, Patil S, Ahn SH, et al. Regional comparison of multiphase computed tomographic angiography and computed tomographic perfusion for prediction of tissue fate in ischemic stroke. Stroke. 2017; 48:939–945.69. Almekhlafi MA, Kunz WG, McTaggart RA, Jayaraman MV, Najm M, Ahn SH, et al. Imaging triage of patients with late-window (6-24 hours) acute ischemic stroke: a comparative study using multiphase CT angiography versus CT perfusion. AJNR Am J Neuroradiol. 2020; 41:129–133.70. Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015; 372:1019–1030.71. Dundamadappa S, Iyer K, Agrawal A, Choi DJ. Multiphase CT angiography: a useful technique in acute stroke imaging—collaterals and beyond. AJNR Am J Neuroradiol. 2021; 42:221–227.72. Yang CY, Chen YF, Lee CW, Huang A, Shen Y, Wei C, et al. Multiphase CT angiography versus single-phase CT angiography: comparison of image quality and radiation dose. AJNR Am J Neuroradiol. 2008; 29:1288–1295.73. Martins N, Aires A, Mendez B, Boned S, Rubiera M, Tomasello A, et al. Ghost infarct core and admission computed tomography perfusion: redefining the role of neuroimaging in acute ischemic stroke. Interv Neurol. 2018; 7:513–521.74. Hoving JW, Marquering HA, Majoie CBLM, Yassi N, Sharma G, Liebeskind DS, et al. Volumetric and spatial accuracy of computed tomography perfusion estimated ischemic core volume in patients with acute ischemic stroke. Stroke. 2018; 49:2368–2375.75. Demeestere J, Wouters A, Christensen S, Lemmens R, Lansberg MG. Review of perfusion imaging in acute ischemic stroke: from time to tissue. Stroke. 2020; 51:1017–1024.76. Austein F, Riedel C, Kerby T, Meyne J, Binder A, Lindner T, et al. Comparison of perfusion CT software to predict the final infarct volume after thrombectomy. Stroke. 2016; 47:2311–2317.77. Thomalla G, Simonsen CZ, Boutitie F, Andersen G, Berthezene Y, Cheng B, et al. MRI-guided thrombolysis for stroke with unknown time of onset. N Engl J Med. 2018; 379:611–622.78. Ma H, Campbell BCV, Parsons MW, Churilov L, Levi CR, Hsu C, et al. Thrombolysis guided by perfusion imaging up to 9 hours after onset of stroke. N Engl J Med. 2019; 380:1795–1803.79. Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015; 372:2285–2295.80. Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015; 372:1009–1018.81. Amukotuwa SA, Wu A, Zhou K, Page I, Brotchie P, Bammer R. Distal medium vessel occlusions can be accurately and rapidly detected using Tmax maps. Stroke. 2021; 52:3308–3317.82. Becks MJ, Manniesing R, Vister J, Pegge SAH, Steens SCA, van Dijk EJ, et al. Brain CT perfusion improves intracranial vessel occlusion detection on CT angiography. J Neuroradiol. 2019; 46:124–129.83. Kerleroux B, Janot K, Dargazanli C, Daly-Eraya D, Ben-Hassen W, Zhu F, et al. Perfusion imaging to select patients with large ischemic core for mechanical thrombectomy. J Stroke. 2020; 22:225–233.84. Chiu FY, Teng MM, Kao YH, Chen YD, Luo CB, Chang FC, et al. Selection of arterial input function for postprocessing of cerebral CT perfusion in chronic unilateral high-grade stenosis or occlusion of the carotid or middle cerebral artery. Acad Radiol. 2012; 19:8–16.85. Marchal G, Young AR, Baron JC. Early postischemic hyperperfusion: pathophysiologic insights from positron emission tomography. J Cereb Blood Flow Metab. 1999; 19:467–482.86. Burke JF, Kerber KA, Iwashyna TJ, Morgenstern LB. Wide variation and rising utilization of stroke magnetic resonance imaging: data from 11 states. Ann Neurol. 2012; 71:179–185.87. Brazzelli M, Sandercock PA, Chappell FM, Celani MG, Righetti E, Arestis N, et al. Magnetic resonance imaging versus computed tomography for detection of acute vascular lesions in patients presenting with stroke symptoms. Cochrane Database Syst Rev. 2009; 4:CD007424.88. Matsumoto K, Lo EH, Pierce AR, Wei H, Garrido L, Kowall NW. Role of vasogenic edema and tissue cavitation in ischemic evolution on diffusion-weighted imaging: comparison with multiparameter MR and immunohistochemistry. AJNR Am J Neuroradiol. 1995; 16:1107–1115.89. Campbell BC, Purushotham A, Christensen S, Desmond PM, Nagakane Y, Parsons MW, et al. The infarct core is well represented by the acute diffusion lesion: sustained reversal is infrequent. J Cereb Blood Flow Metab. 2012; 32:50–56.90. Edlow BL, Hurwitz S, Edlow JA. Diagnosis of DWI-negative acute ischemic stroke: a meta-analysis. Neurology. 2017; 89:256–262.91. Labeyrie MA, Turc G, Hess A, Hervo P, Mas JL, Meder JF, et al. Diffusion lesion reversal after thrombolysis: a MR correlate of early neurological improvement. Stroke. 2012; 43:2986–2991.92. Kranz PG, Eastwood JD. Does diffusion-weighted imaging represent the ischemic core? An evidence-based systematic review. AJNR Am J Neuroradiol. 2009; 30:1206–1212.93. Makkat S, Vandevenne JE, Verswijvel G, Ijsewijn T, Grieten M, Palmers Y, et al. Signs of acute stroke seen on fluid-attenuated inversion recovery MR imaging. AJR Am J Roentgenol. 2002; 179:237–243.94. Thomalla G, Cheng B, Ebinger M, Hao Q, Tourdias T, Wu O, et al. DWI-FLAIR mismatch for the identification of patients with acute ischaemic stroke within 4·5 h of symptom onset (PREFLAIR): a multicentre observational study. Lancet Neurol. 2011; 10:978–986.95. Emeriau S, Serre I, Toubas O, Pombourcq F, Oppenheim C, Pierot L. Can diffusion-weighted imaging-fluid-attenuated inversion recovery mismatch (positive diffusion-weighted imaging/negative fluid-attenuated inversion recovery) at 3 tesla identify patients with stroke at <4.5 hours? Stroke. 2013; 44:1647–1651.96. Cho AH, Sohn SI, Han MK, Lee DH, Kim JS, Choi CG, et al. Safety and efficacy of MRI-based thrombolysis in unclear-onset stroke. A preliminary report. Cerebrovasc Dis. 2008; 25:572–579.97. Fischer U, Branca M, Bonati LH, Carrera E, Vargas MI, Platon A, et al. Magnetic resonance imaging or computed tomography for suspected acute stroke: association of admission image modality with acute recanalization therapies, workflow metrics, and outcomes. Ann Neurol. 2022; 92:184–194.98. Kidwell CS, Chalela JA, Saver JL, Starkman S, Hill MD, Demchuk AM, et al. Comparison of MRI and CT for detection of acute intracerebral hemorrhage. JAMA. 2004; 292:1823–1830.99. Arnould MC, Grandin CB, Peeters A, Cosnard G, Duprez TP. Comparison of CT and three MR sequences for detecting and categorizing early (48 hours) hemorrhagic transformation in hyperacute ischemic stroke. AJNR Am J Neuroradiol. 2004; 25:939–944.100. Liebeskind DS, Sanossian N, Yong WH, Starkman S, Tsang MP, Moya AL, et al. CT and MRI early vessel signs reflect clot composition in acute stroke. Stroke. 2011; 42:1237–1243.101. Mohammaden MH, Haussen DC, Perry da Camara C, Pisani L, Olive Gadea M, Al-Bayati AR, et al. Hyperdense vessel sign as a potential guide for the choice of stent retriever versus contact aspiration as first-line thrombectomy strategy. J Neurointerv Surg. 2021; 13:599–604.102. Suh HI, Hong JM, Lee KS, Han M, Choi JW, Kim JS, et al. Imaging predictors for atherosclerosis-related intracranial large artery occlusions in acute anterior circulation stroke. J Stroke. 2016; 18:352–354.103. Lee JS, Hong JM, Kim JS. Diagnostic and therapeutic strategies for acute intracranial atherosclerosis-related occlusions. J Stroke. 2017; 19:143–151.104. Menjot de Champfleur N, Saver JL, Goyal M, Jahan R, Diener HC, Bonafe A, et al. Efficacy of stent-retriever thrombectomy in magnetic resonance imaging versus computed tomographic perfusion–selected patients in SWIFT PRIME trial (solitaire FR with the intention for thrombectomy as primary endovascular treatment for acute ischemic stroke). Stroke. 2017; 48:1560–1566.105. Kim JT, Cho BH, Choi KH, Park MS, Kim BJ, Park JM, et al. Magnetic resonance imaging versus computed tomography angiography based selection for endovascular therapy in patients with acute ischemic stroke. Stroke. 2019; 50:365–372.106. Eskey CJ, Meyers PM, Nguyen TN, Ansari SA, Jayaraman M, McDougall CG, et al. Indications for the performance of intracranial endovascular neurointerventional procedures: a scientific statement from the American Heart Association. Circulation. 2018; 137:e661–e689.107. Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018; 378:11–21.108. Yamagami H, Hayakawa M, Inoue M, Iihara K, Ogasawara K, Toyoda K, et al. Guidelines for mechanical thrombectomy in japan, the fourth edition, March 2020: a guideline from the Japan Stroke Society, the Japan Neurosurgical Society, and the Japanese Society for Neuroendovascular Therapy. Neurol Med Chir (Tokyo). 2021; 61:163–192.109. Siegler JE, Qureshi MM, Nogueira RG, Tanaka K, Nagel S, Michel P, Vigilante N, Ribo M, Yamagami H, Yoshimura S, et al. Endovascular versus medical management for late-window anterior large vessel occlusion with pre-stroke disability: Patient-level analysis from the CLEAR and RESCUE-Japan studies. Neurology. 2022; Nov. 4. [Epub]. https://doi.org/10.1212/WNL.0000000000201543.110. Kim Y, Lee S, Abdelkhaleq R, Lopez-Rivera V, Navi B, Kamel H, et al. Utilization and availability of advanced imaging in patients with acute ischemic stroke. Circ Cardiovasc Qual Outcomes. 2021; 14:e006989.111. Leslie-Mazwi TM, Hamilton S, Mlynash M, Patel AB, Schwamm LH, Lansberg MG, et al. DEFUSE 3 non-DAWN patients. Stroke. 2019; 50:618–625.112. Siegler JE, Messé SR, Sucharew H, Kasner SE, Mehta T, Arora N, et al. Thrombectomy in DAWN- and DEFUSE-3-ineligible patients: a subgroup analysis from the best prospective cohort study. Neurosurgery. 2020; 86:E156–E163.113. Nguyen TN, Raymond J, Nogueira RG, Fischer U, Siegler JE. The problem of restrictive thrombectomy trial eligibility criteria. Stroke. 2022; 53:2988–2990.114. Jadhav AP, Desai SM, Kenmuir CL, Rocha M, Starr MT, Molyneaux BJ, et al. Eligibility for endovascular trial enrollment in the 6- to 24-hour time window: analysis of a single comprehensive stroke center. Stroke. 2018; 49:1015–1017.115. Desai SM, Rocha M, Molyneaux BJ, Starr M, Kenmuir CL, Gross BA, et al. Thrombectomy 6-24 hours after stroke in trial ineligible patients. J Neurointerv Surg. 2018; 10:1033–1037.116. Ducroux C, Khoury N, Lecler A, Blanc R, Chetrit A, Redjem H, et al. Application of the DAWN clinical imaging mismatch and DEFUSE 3 selection criteria: benefit seems similar but restrictive volume cut-offs might omit potential responders. Eur J Neurol. 2018; 25:1093–1099.117. Santos T, Carvalho A, Cunha AA, Rodrigues M, Gregório T, Paredes L, et al. NCCT and CTA-based imaging protocol for endovascular treatment selection in late presenting or wakeup strokes. J Neurointerv Surg. 2019; 11:200–203.118. DiBiasio EL, Jayaraman MV, Goyal M, Yaghi S, Tung E, Hidlay DT, et al. Dismantling the ability of CT and MRI to identify the target mismatch profile in patients with anterior circulation large vessel occlusion beyond six hours from symptom onset. Emerg Radiol. 2019; 26:401–408.119. Pirson FAVA, Hinsenveld WH, Goldhoorn RB, Staals J, de Ridder IR, van Zwam WH; MR CLEAN-LATE investigators. MR CLEAN-LATE, a multicenter randomized clinical trial of endovascular treatment of acute ischemic stroke in the Netherlands for late arrivals: study protocol for a randomized controlled trial. Trials. 2021; 22:160.120. Almekhlafi MA, Thornton J, Casetta I, Goyal M, Nannoni S, Herlihy D, et al. Stroke imaging prior to thrombectomy in the late window: results from a pooled multicentre analysis. J Neurol Neurosurg Psychiatry. 2022; 93:468–474.121. Almekhlafi MA, Kunz WG, Menon BK, McTaggart RA, Jayaraman MV, Baxter BW, et al. Imaging of patients with suspected large-vessel occlusion at primary stroke centers: available modalities and a suggested approach. AJNR Am J Neuroradiol. 2019; 40:396–400.122. Demeestere J, Scheldeman L, Cornelissen SA, Heye S, Wouters A, Dupont P, et al. Alberta stroke program early CT score versus computed tomographic perfusion to predict functional outcome after successful reperfusion in acute ischemic stroke. Stroke. 2018; 49:2361–2367.123. Dhillon PS, Butt W, Podlasek A, Barrett E, McConachie N, Lenthall R, et al. Endovascular thrombectomy beyond 24 hours from ischemic stroke onset: a propensity score matched cohort study. J Neurointerv Surg. 2022; Feb. 15. [Epub]. https://doi.org/10.1136/neurintsurg-2021-018591.124. Christensen S, Mlynash M, Kemp S, Yennu A, Heit JJ, Marks MP, et al. Persistent target mismatch profile >24 hours after stroke onset in DEFUSE 3. Stroke. 2019; 50:754–757.125. Stanton RJ, Wang LL, Smith MS, Aziz Y, Zhang B, Grossman AW, et al. Differences in automated perfusion software: do they matter clinically? Stroke Vasc Interv Neurol. 2022; 2:e000424.126. Murray NM, Unberath M, Hager GD, Hui FK. Artificial intelligence to diagnose ischemic stroke and identify large vessel occlusions: a systematic review. J Neurointerv Surg. 2020; 12:156–164.127. Grunwald IQ, Kulikovski J, Reith W, Gerry S, Namias R, Politi M, et al. Collateral automation for triage in stroke: evaluating automated scoring of collaterals in acute stroke on computed tomography scans. Cerebrovasc Dis. 2019; 47:217–222.128. Kamel H, Navi BB, Parikh NS, Merkler AE, Okin PM, Devereux RB, et al. Machine learning prediction of stroke mechanism in embolic strokes of undetermined source. Stroke. 2020; 51:e203–e210.129. Brugnara G, Neuberger U, Mahmutoglu MA, Foltyn M, Herweh C, Nagel S, et al. Multimodal predictive modeling of endovascular treatment outcome for acute ischemic stroke using machine-learning. Stroke. 2020; 51:3541–3551.130. Lin X, Zheng X, Zhang J, Cui X, Zou D, Zhao Z, et al. Machine learning to predict futile recanalization of large vessel occlusion before and after endovascular thrombectomy. Front Neuro. 2022; 13:909403.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neuroimaging in Randomized, Multi-Center Clinical Trials of Endovascular Treatment for Acute Ischemic Stroke: A Systematic Review
- Fast MRI in Acute Ischemic Stroke: Applications of MRI Acceleration Techniques for MR-Based Comprehensive Stroke Imaging
- Intravenous Thrombolysis and Endovascular Thrombectomy in Acute Ischemic Stroke with Minor Symptom
- Endovascular Treatment of Acute Ischemic Stroke
- Reperfusion therapy in acute ischemic stroke