Korean J Radiol.
2019 Jul;20(7):1186-1194. 10.3348/kjr.2018.0921.
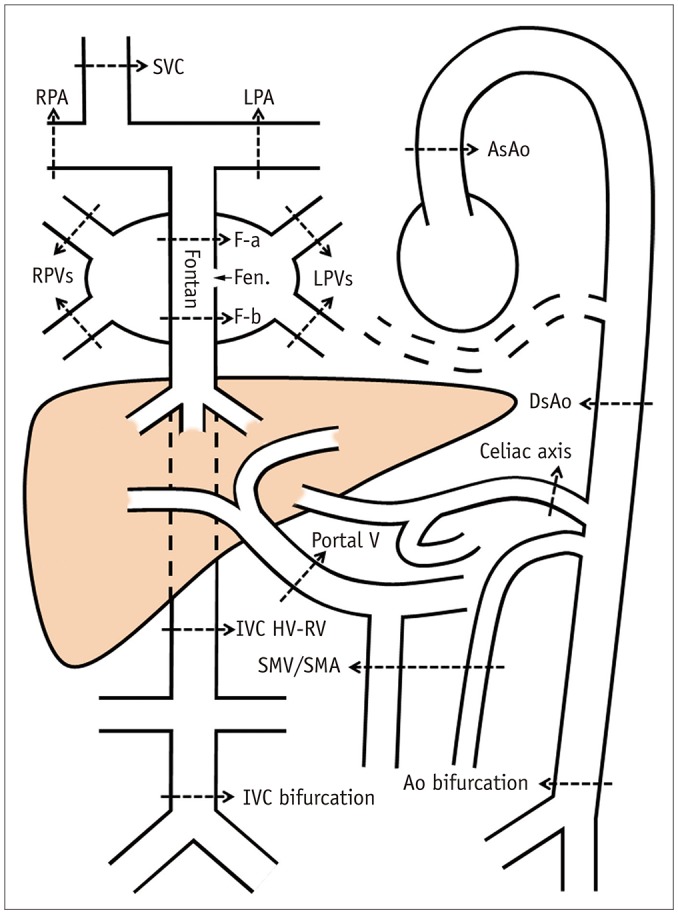
Magnetic Resonance Imaging Assessment of Blood Flow Distribution in Fenestrated and Completed Fontan Circulation with Special Emphasis on Abdominal Blood Flow
- Affiliations
-
- 1Department of Diagnostic Imaging, The Hospital for Sick Children, University of Toronto, Toronto, Canada. shi-joon.yoo@sickkids.ca
- 2The Labatt Family Heart Center, The Hospital for Sick Children, University of Toronto, Toronto, Canada.
- 3Division of Gastroenterology, Hepatology & Nutrition, Department of Paediatrics, The Hospital for Sick Children, University of Toronto, Toronto, Canada.
- 4Department of Medical Imaging, University Health Network, University of Toronto, Toronto, Canada.
- KMID: 2467029
- DOI: http://doi.org/10.3348/kjr.2018.0921
Abstract
OBJECTIVE
To investigate the regional flow distribution in patients with Fontan circulation by using magnetic resonance imaging (MRI).
MATERIALS AND METHODS
We identified 39 children (18 females and 21 males; mean age, 9.3 years; age range, 3.3-17.0 years) with Fontan circulation in whom flow volumes across the thoracic and abdominal arteries and veins were measured by using MRI. The patients were divided into three groups: fenestrated Fontan circulation group with MRI performed under general anesthesia (GA) (Group 1, 15 patients; average age, 5.9 years), completed Fontan circulation group with MRI performed under GA (Group 2, 6 patients; average age, 8.7 years), and completed Fontan circulation group with MRI performed without GA (Group 3, 18 patients; average age, 12.5 years). The patient data were compared with the reference ranges in healthy controls.
RESULTS
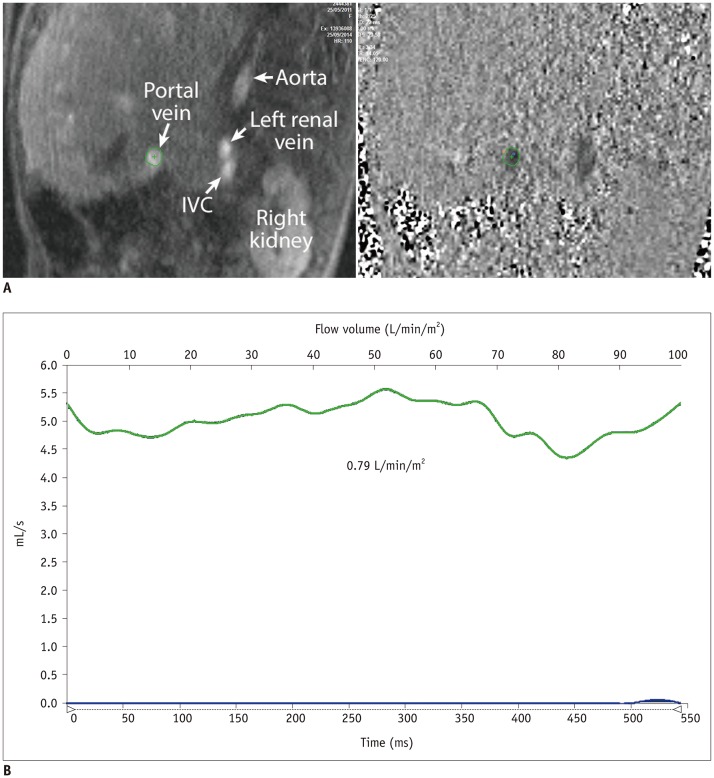
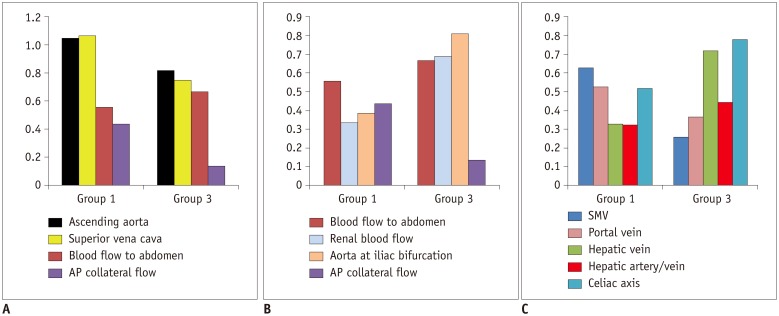
In comparison with the controls, Group 1 showed normal cardiac output (3.92 ± 0.40 vs. 3.72 ± 0.69 L/min/m2, p = 0.30), while Group 3 showed decreased cardiac output (3.24 ± 0.71 vs. 3.96 ± 0.64 L/min/m2, p = 0.003). Groups 1 and 3 showed reduced abdominal flow (1.21 ± 0.28 vs. 2.37 ± 0.45 L/min/m2, p < 0.001 and 1.89 ± 0.39 vs. 2.64 ± 0.38 L/min/m2, p < 0.001, respectively), which was mainly due to the diversion of the cardiac output to the aortopulmonary collaterals in Group 1 and the reduced cardiac output in Group 3. Superior mesenteric and portal venous flows were more severely reduced in Group 3 than in Group 1 (ratios between the flow volumes of the patients and healthy controls was 0.26 and 0.37 in Group 3 and 0.63 and 0.53 in Group 1, respectively). Hepatic arterial flow was decreased in Group 1 (0.11 ± 0.22 vs. 0.34 ± 0.38 L/min/m2, p = 0.04) and markedly increased in Group 3 (0.38 ± 0.22 vs. −0.08 ± 0.29 L/min/m2, p < 0.0001). Group 2 showed a mixture of the patterns seen in Groups 1 and 3.
CONCLUSION
Fontan circulation is associated with reduced abdominal flow, which can be attributed to reduced cardiac output and portal venous return in completed Fontan circulation, and diversion of the cardiac output to the aortopulmonary collaterals in fenestrated Fontan circulation.
Keyword
MeSH Terms
Figure
Reference
-
1. Rychik J. The relentless effects of the Fontan paradox. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2016; 19:37–43. PMID: 27060041.
Article2. Gewillig M, Goldberg DJ. Failure of the fontan circulation. Heart Fail Clin. 2014; 10:105–116. PMID: 24275298.
Article3. Bridges ND, Jonas RA, Mayer JE, Flanagan MF, Keane JF, Castaneda AR. Bidirectional cavopulmonary anastomosis as interim palliation for high-risk Fontan candidates. Early results. Circulation. 1990; 82(5 Suppl):IV170–IV176. PMID: 1699686.4. Bridges ND, Lock JE, Castaneda AR. Baffle fenestration with subsequent transcatheter closure. Modification of the Fontan operation for patients at increased risk. Circulation. 1990; 82:1681–1689. PMID: 2225370.
Article5. de Leval MR. Evolution of the Fontan-Kreutzer procedure. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2010; 13:91–95. PMID: 20307869.
Article6. Kiesewetter CH, Sheron N, Vettukattill JJ, Hacking N, Stedman B, Millward-Sadler H, et al. Hepatic changes in the failing Fontan circulation. Heart. 2007; 93:579–584. PMID: 17005713.
Article7. Wu FM, Kogon B, Earing MG, Aboulhosn JA, Broberg CS, John AS, et al. Alliance for Adult Research in Congenital Cardiology (AARCC) Investigators. Liver health in adults with Fontan circulation: a multicenter cross-sectional study. J Thorac Cardiovasc Surg. 2017; 153:656–664. PMID: 27955914.
Article8. Meadows J, Jenkins K. Protein-losing enteropathy: integrating a new disease paradigm into recommendations for prevention and treatment. Cardiol Young. 2011; 21:363–377. PMID: 21349233.
Article9. Schumacher KR, Stringer KA, Donohue JE, Yu S, Shaver A, Caruthers RL, et al. Fontan-associated protein-losing enteropathy and plastic bronchitis. J Pediatr. 2015; 166:970–977. PMID: 25661406.
Article10. Muthusami P, Yoo SJ, Chaturvedi R, Gill N, Windram J, Schantz D, et al. Splanchnic, thoracoabdominal, and cerebral blood flow volumes in healthy children and young adults in fasting and postprandial states: determining reference ranges by using phase-contrast MR imaging. Radiology. 2017; 285:231–241. PMID: 28530848.
Article11. Grosse-Wortmann L, Al-Otay A, Yoo SJ. Aortopulmonary collaterals after bidirectional cavopulmonary connection or Fontan completion: quantification with MRI. Circ Cardiovasc Imaging. 2009; 2:219–225. PMID: 19808596.12. Fogel MA, Li C, Wilson F, Pawlowski T, Nicolson SC, Montenegro LM, et al. Relationship of cerebral blood flow to aortic-to-pulmonary collateral/shunt flow in single ventricles. Heart. 2015; 101:1325–1331. PMID: 26048877.
Article13. Eyskens B, Mertens L, Kuzo R, De Jaegere T, Lawrenson J, Dymarkowski S, et al. The ratio of flow in the superior and inferior caval veins after construction of a bidirectional cavopulmonary anastomosis in children. Cardiol Young. 2003; 13:123–130. PMID: 12887067.
Article14. Whitehead KK, Gillespie MJ, Harris MA, Fogel MA, Rome JJ. Noninvasive quantification of systemic-to-pulmonary collateral flow: a major source of inefficiency in patients with superior cavopulmonary connections. Circ Cardiovasc Imaging. 2009; 2:405–411. PMID: 19808629.15. Prakash A, Rathod RH, Powell AJ, McElhinney DB, Banka P, Geva T. Relation of systemic-to-pulmonary artery collateral flow in single ventricle physiology to palliative stage and clinical status. Am J Cardiol. 2012; 109:1038–1045. PMID: 22221948.
Article16. Grosse-Wortmann L, Dragulescu A, Drolet C, Chaturvedi R, Kotani Y, Mertens L, et al. Determinants and clinical significance of flow via the fenestration in the Fontan pathway: a multimodality study. Int J Cardiol. 2013; 168:811–817. PMID: 23164583.
Article17. Eipel C, Abshagen K, Vollmar B. Regulation of hepatic blood flow: the hepatic arterial buffer response revisited. World J Gastroenterol. 2010; 16:6046–6057. PMID: 21182219.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Fenestrated Stent Graft Repair of Abdominal Aortic Aneurysm: Hemodynamic Analysis of the Effect of Fenestrated Stents on the Renal Arteries
- Four-Dimensional Flow Magnetic Resonance Imaging for Cardiovascular Imaging: from Basic Concept to Clinical Application
- Renal Blood Flow in Chronic Glomerulonephritis
- Computed Tomography in the Evaluation of Fontan Circulation
- Estimation of Occluded Sites and Flow Dynamics in Internal Carotid Artery Obstruction Using Routine Cerebral Magnetic Resonance Imaging and Transcranial Doppler