Lab Med Online.
2020 Jan;10(1):25-32. 10.3343/lmo.2020.10.1.25.
Performance Evaluation of the CS-5100 Coagulation Analyzer for Special Coagulation Parameters
- Affiliations
-
- 1Department of Laboratory Medicine, Soonchunhyang University Cheonan Hospital, Soonchunhyang University College of Medicine, Cheonan, Korea.
- 2Department of Laboratory Medicine, University of Ulsan College of Medicine and Asan Medical Center, Seoul, Korea. ssjang@amc.seoul.kr
- 3Asan Clinical Research Center, Seoul, Korea.
- KMID: 2466762
- DOI: http://doi.org/10.3343/lmo.2020.10.1.25
Abstract
- BACKGROUND
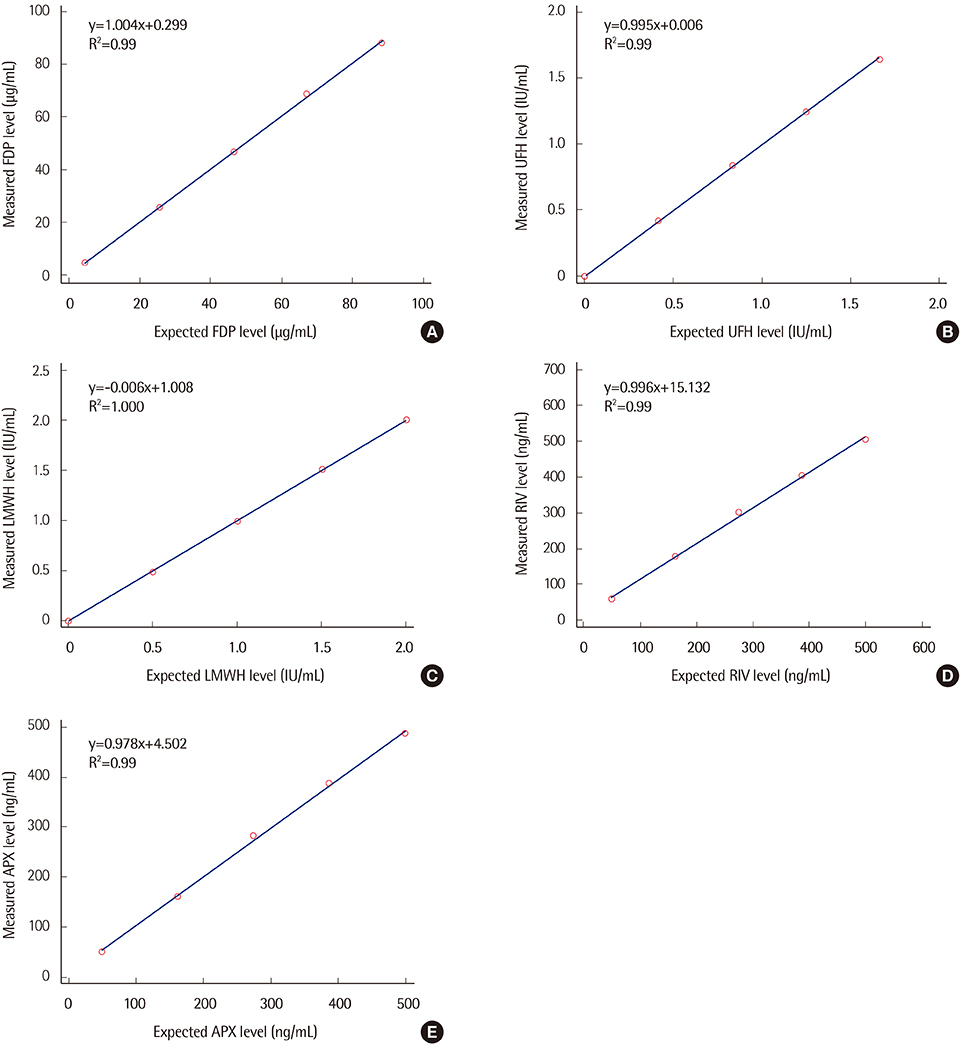
The CS-5100 high-throughput automated coagulometer (Sysmex, Japan) can perform general and special coagulation assays. We evaluated the performance of the CS-5100 coagulometer to measure fibrinogen degradation product (FDP), plasma level of unfractionated heparin (UFH), low molecular weight heparin (LMWH), and direct oral anticoagulants (DOACs).
METHODS
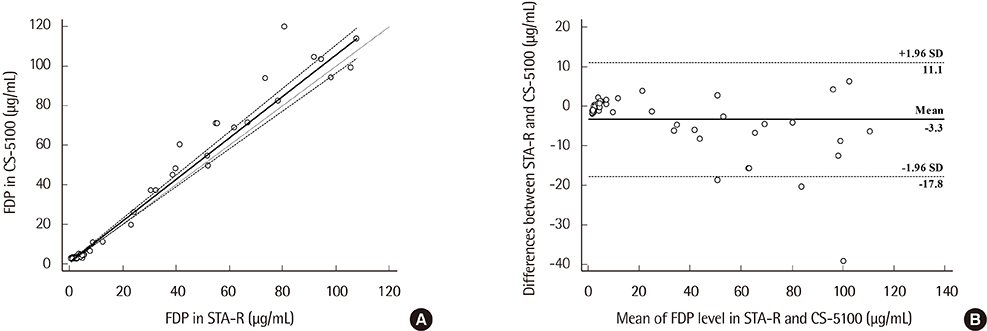
Precision, linearity, and carryover rate of the CS-5100 while measuring FDP, UFH, and LMWH were validated. Precision and linearity were validated for measurements of DOACs. Results of FDP measurement using CS-5100 were compared to those using the currently used STA-R coagulometer (Diagnostica Stago, France). The reference range of FDP was established. All evaluation procedures were in accordance with Clinical and Laboratory Standards Institute guidelines.
RESULTS
CS-5100 displayed good precision, linearity, and carryover rate for measuring FDP, UFH, LMWH, and DOACs. The FDP level measured with the CS-5100 and STA-R coagulometers correlated well. The reference range of FDP with CS-5100 was 0.0 to 3.48 µg/mL.
CONCLUSIONS
The CS-5100 coagulometer has acceptable analytical performance in measuring special coagulation parameters. It is suitable for the reliable measurement of plasma FDP and anticoagulant levels in clinical laboratories.
Keyword
MeSH Terms
Figure
Reference
-
1. Ratzinger F, Schmetterer KG, Haslacher H, Perkmann T, Belik S, Quehenberger P. Evaluation of the automated coagulation analyzer CS-5100 and its utility in high throughput laboratories. Clin Chem Lab Med. 2014; 52:1193–1202.
Article2. Geens T, Vertessen F, Malfait R, Deiteren K, Maes MB. Validation of the Sysmex CS5100 coagulation analyzer and comparison to the Stago STA-R analyzer for routine coagulation parameters. Int J Lab Hematol. 2015; 37:372–381.
Article3. Chen L, Chen Y. Performance evaluation of the Sysmex CS-5100 automated coagulation analyzer. Clin Lab. 2015; 61:653–660.
Article4. Flieder T, Gripp T, Knabbe C, Birschmann I. The Sysmex CS-5100 coagulation analyzer offers comparable analytical performance and excellent throughput capabilities. Pract Lab Med. 2016; 6:38–47.
Article5. Camici GG, Steffel J, Akhmedov A, Schafer N, Baldinger J, Schulz U, et al. Dimethyl sulfoxide inhibits tissue factor expression, thrombus formation, and vascular smooth muscle cell activation: a potential treatment strategy for drug-eluting stents. Circulation. 2006; 114:1512–1521.
Article6. Clinical and Laboratory Standards Institute. Evaluation of precision of quantitative measurement procedures; approved guideline—third edition (EP05-A3). Wayne, PA: Clinical and Laboratory Standards Institute;2014.7. Clinical and Laboratory Standards Institute. Evaluation of the linearity of quantitative measurement procedures: a statistical approach; approved guideline (EP06-A). Wayne, PA: Clinical and Laboratory Standards Institute;2003.8. Clinical and Laboratory Standards Institute. Preliminary evaluation of quantitative clinical laboratory measurement procedures; approved guideline—third edition (EP10-A3-AMD). Wayne, PA: Clinical and Laboratory Standards Institute;2014.9. Clinical and Laboratory Standards Institute. Measurement procedure comparison and bias estimation using patient samples; approved guideline—third edition (EP09-A3). Wayne, PA: Clinical and Laboratory Standards Institute;2013.10. Clinical and Laboratory Standards Institute. Dening, establishing, and verifying reference intervals in the clinical laboratory; approved guideline—third edition (EP28-A3c). Wayne, PA: Clinical and Laboratory Standards Institute;2008.11. McPherson RA, Pincus MR, editors. Henry's clinical diagnosis and management by laboratory methods. 23rd ed. St. Louis: Elsevier;2017.12. Gardiner C, Kitchen S, Dauer RJ, Kottke-Marchant K, Adcock DM. Recommendations for evaluation of coagulation analyzers. Lab Hematol. 2006; 12:32–38.
Article13. Garcia DA, Baglin TP, Weitz JI, Samama MM. Parenteral anticoagulants: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012; 141:e24S–e43S.14. Vandiver JW, Vondracek TG. Antifactor Xa levels versus activated partial thromboplastin time for monitoring unfractionated heparin. Pharmacotherapy. 2012; 32:546–558.
Article15. Baluwala I, Favaloro EJ, Pasalic L. Therapeutic monitoring of unfractionated heparin - trials and tribulations. Expert Rev Hematol. 2017; 10:595–605.
Article16. Gouin-Thibault I, Pautas E, Siguret V. Safety prole of different low-molecular weight heparins used at therapeutic dose. Drug Saf. 2005; 28:333–349.
Article17. Thomas O, Lybeck E, Strandberg K, Tynngard N, Schott U. Monitoring low molecular weight heparins at therapeutic levels: dose-responses of, and correlations and differences between aPTT, anti-factor Xa and thrombin generation assays. PLoS One. 2015; 10:e0116835.
Article18. Samama MM, Contant G, Spiro TE, Perzborn E, Flem LL, Guinet C, et al. Evaluation of the prothrombin time for measuring rivaroxaban plasma concentrations using calibrators and controls: results of a multicenter eld trial. Clin Appl Thromb Hemost. 2012; 18:150–158.
Article19. Mueck W, Eriksson BI, Bauer KA, Borris L, Dahl OE, Fisher WD, et al. Population pharmacokinetics and pharmacodynamics of rivaroxaban--an oral, direct factor Xa inhibitor--in patients undergoing major orthopaedic surgery. Clin Pharmacokinet. 2008; 47:203–216.
Article20. Perzborn E, Strassburger J, Wilmen A, Pohlmann J, Roehrig S, Schlemmer KH, et al. In vitro and in vivo studies of the novel antithrombotic agent BAY 59-7939--an oral, direct Factor Xa inhibitor. J Thromb Haemost. 2005; 3:514–521.
Article21. Kubitza D, Becka M, Roth A, Mueck W. Absence of clinically relevant interactions between rivaroxaban--an oral, direct Factor Xa inhibitor-- and digoxin or atorvastatin in healthy subjects. J Int Med Res. 2012; 40:1688–1707.
Article22. Douxls J, Tamigniau A, Chatelain B, Gofnet C, Dogne JM, Mullier F. Measurement of non-VKA oral anticoagulants versus classic ones: the appropriate use of hemostasis assays. Thromb J. 2014; 12:24.
Article23. Lippi G, Favaloro EJ. Recent guidelines and recommendations for laboratory assessment of the direct oral anticoagulants (DOACs): is there consensus? Clin Chem Lab Med. 2015; 53:185–197.
Article24. Adcock DM, Gosselin R. Direct oral anticoagulants (DOACs) in the laboratory: 2015 review. Thromb Res. 2015; 136:7–12.
Article25. Harenberg J, Erdle S, Marx S, Kramer R. Determination of rivaroxaban in human plasma samples. Semin Thromb Hemost. 2012; 38:178–184.
Article26. Tripodi A, Chantarangkul V, Guinet C, Samama MM. The International Normalized Ratio calibrated for rivaroxaban has the potential to normalize prothrombin time results for rivaroxaban-treated patients: results of an in vitro study. J Thromb Haemost. 2011; 9:226–228.
Article27. Blech S, Ebner T, Ludwig-Schwellinger E, Stangier J, Roth W. The metabolism and disposition of the oral direct thrombin inhibitor, dabigatran, in humans. Drug Metab Dispos. 2008; 36:386–399.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of Automated Platelet Aggregation Test Using a Sysmex CS-5100 Analyzer
- Analytical Performance of INNOVANCE Free Protein S Antigen on Sysmex CS-5100
- Performance Evaluation and Local International Sensitivity Index Verification Using Automated Coagulation Analyzer Coapresta 2000
- Influence of Blood Coagulation Factors on Thromboelastographic Parameters in Healthy Adults
- Performance Evaluation of the Automated Coagulation Analyzer Coapresta 2000