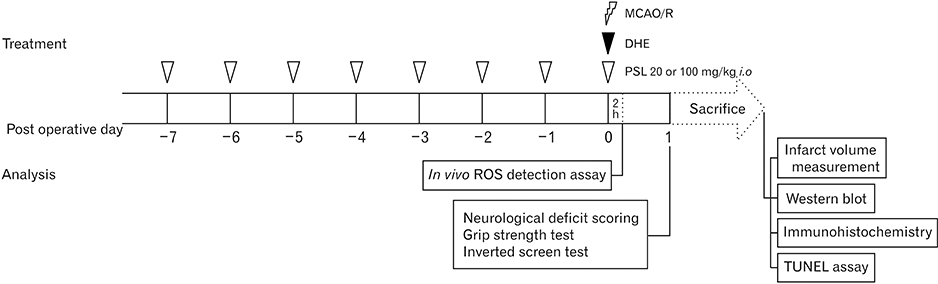
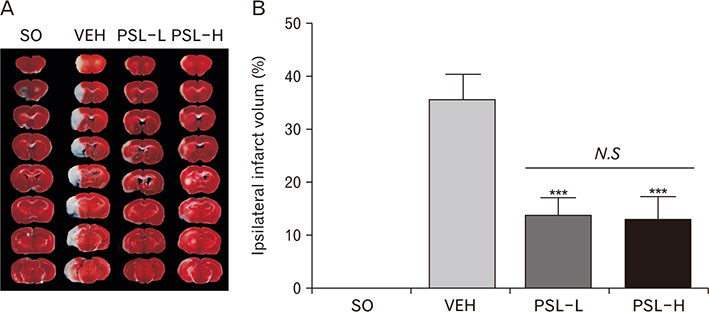
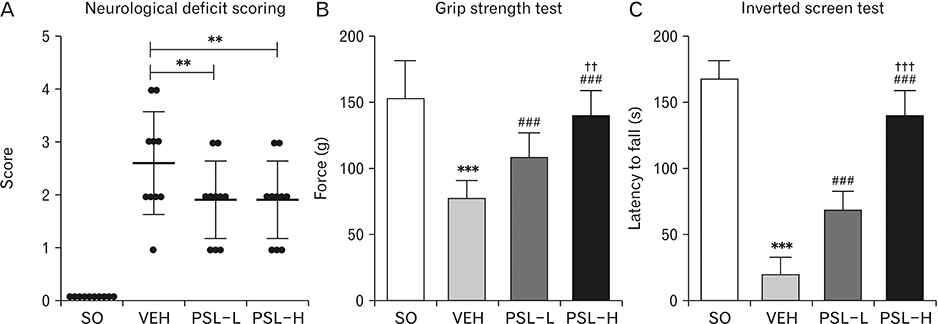
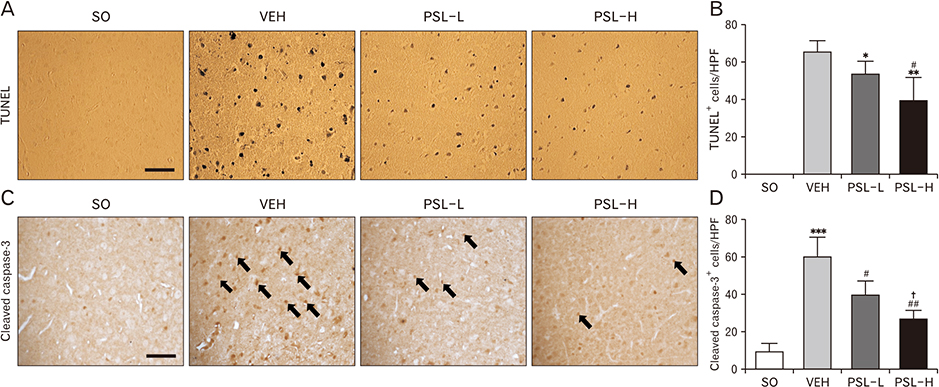
Anat Cell Biol.
2019 Dec;52(4):486-497. 10.5115/acb.19.141.
Platycarya strobilacea leaf extract protects mice brain with focal cerebral ischemia by antioxidative property
- Affiliations
-
- 1Department of Anatomy, Konyang University College of Medicine, Daejeon, Korea. jjzzy@konyang.ac.kr
- 2Lifetree Co., Ltd., Suwon, Korea.
- 3Department of Herbal Health and Pharmacy, Joongbu University College of Health and Welfare, Geumsan, Korea. esdoh@joongbu.ac.kr
- KMID: 2466703
- DOI: http://doi.org/10.5115/acb.19.141
Abstract
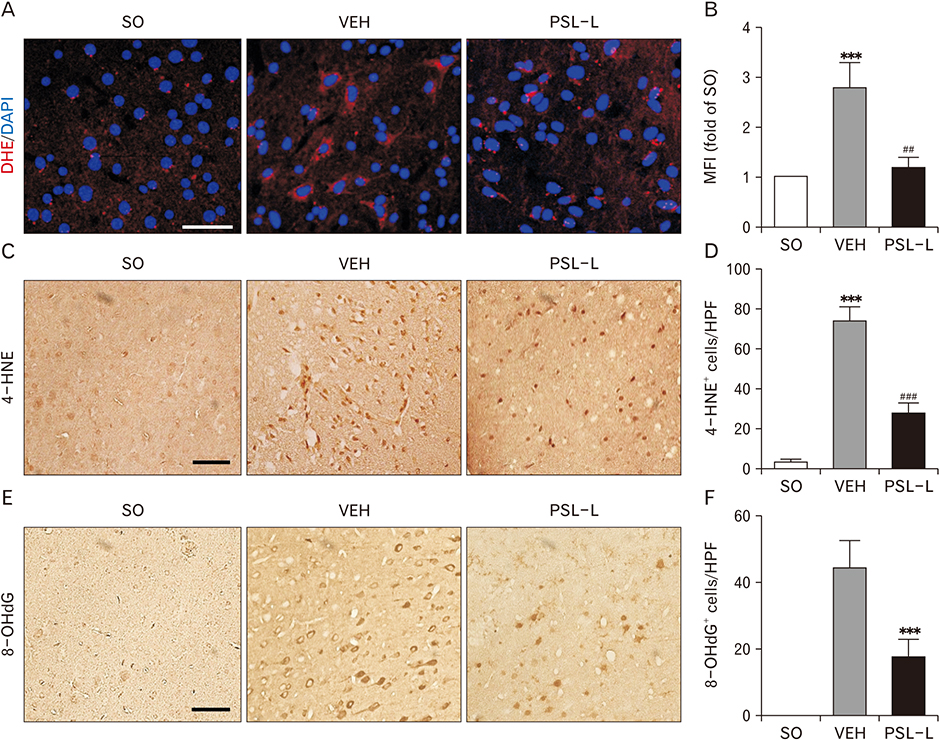
- The leaf extract of Platycarya strobilacea (PSL) has long been recognized as possessing various health-promoting activities. However, information on its possible protective effects against ischemic stroke is currently lacking. Here, using a mouse model of focal cerebral ischemia (fCI), we studied the protective potential of an oral supplement of PSL. Mice were randomly divided into four groups: SO, a group subjected to a sham-operation; VEH, pretreated with distilled water and subjected to middle cerebral artery occlusion and reperfusion (MCAO/R); PSL-L and PSL-H, pretreated with low (20 mg/kg) and high (100 mg/kg) doses of PSL, respectively, and subjected to the MCAO/R procedure. PSL was administered via an oral route daily for 8 days prior to surgery. We then measured the infarct volumes and sensorimotor deficits and studied the underlying antioxidant mechanisms by quantifying apoptosis, reactive oxygen species (ROS) generation, oxidative damages, and antioxidant enzymes in the ischemic cortex. The results showed a marked attenuation in infarct volume and sensorimotor deficits in both the PSL-L and PSL-H groups when compared with VEH. The terminal deoxynucleotidyl transferase dUTP nick end labeling and the immunohistochemical detection of the cleaved caspase-3 revealed that PSL could reduce cellular apoptosis in the ischemic lesion in a dose-dependent manner. The dihydroethidium-fluorescence, 4-hydroxynonenal, and 8-hydroxyl-2"²-deoxyguanosine immunoreactivities in the ischemic lesion were markedly attenuated in the PSL-L group compared with the VEH group, indicating that PSL could attenuate ROS generation and the associated oxidative damage in the ischemic cortex. Finally, western blot results indicated that PSL can upregulate levels of heme oxygenase-1 (HO-1), an antioxidant enzyme, in the lesion area. Together, these results suggest that PSL can exert protective effects against fCI, and the mechanism may involve HO-1 upregulation.
Keyword
MeSH Terms
Figure
Reference
-
1. Bonita R, Beaglehole R, Asplund K. The worldwide problem of stroke. Curr Opin Neurol. 1994; 7:5–10.2. Lee RH, Lee MH, Wu CY, Couto E Silva A, Possoit HE, Hsieh TH, Minagar A, Lin HW. Cerebral ischemia and neuroregeneration. Neural Regen Res. 2018; 13:373–385.3. Lawson TR, Brown IE, Westerkam DL, Blackhurst DW, Sternberg S, Leacock R, Nathaniel TI. Tissue plasminogen activator (rt-PA) in acute ischemic stroke: outcomes associated with ambulation. Restor Neurol Neurosci. 2015; 33:301–308.4. Tekle WG, Chaudhry SA, Hassan AE, Peacock JM, Lakshminarayan K, Tsai A, Luepker R, Anderson DC, Qureshi AI. Utilization of intravenous thrombolysis in 3-4.5 hours: analysis of the Minnesota stroke registry. Cerebrovasc Dis. 2012; 34:400–405.5. Li W, Suwanwela NC, Patumraj S. Curcumin prevents reperfusion injury following ischemic stroke in rats via inhibition of NF κB, ICAM-1, MMP-9 and caspase-3 expression. Mol Med Rep. 2017; 16:4710–4720.6. He X, Deng FJ, Ge JW, Yan XX, Pan AH, Li ZY. Effects of total saponins of Panax notoginseng on immature neuroblasts in the adult olfactory bulb following global cerebral ischemia/reperfusion. Neural Regen Res. 2015; 10:1450–1456.7. Sunwoo YY, Park SI, Chung YA, Lee J, Park MS, Jang KS, Maeng LS, Jang DK, Im R, Jung YJ, Park SA, Kang ES, Kim MW, Han YM. A pilot study for the neuroprotective effect of Gongjin-dan on transient middle cerebral artery occlusion-induced ischemic rat brain. Evid Based Complement Alternat Med. 2012; 2012:682720.8. Lu W, Xv L, Wen J. Protective effect of extract of the Camellia japonica L. on cerebral ischemia-reperfusion injury in rats. Arq Neuropsiquiatr. 2019; 77:39–46.9. Yang H, Zhang A, Zhang Y, Ma S, Wang C. Resveratrol pretreatment protected against cerebral ischemia/reperfusion injury in rats via expansion of t regulatory cells. J Stroke Cerebrovasc Dis. 2016; 25:1914–1921.10. Yan RY, Wang SJ, Yao GT, Liu ZG, Xiao N. The protective effect and its mechanism of 3-n-butylphthalide pretreatment on cerebral ischemia reperfusion injury in rats. Eur Rev Med Pharmacol Sci. 2017; 21:5275–5282.11. Landete JM. Ellagitannins, ellagic acid and their derived metabolites: a review about source, metabolism, functions and health. Food Res Int. 2011; 44:1150–1160.12. Fang F, Qin Y, Qi L, Fang Q, Zhao L, Chen S, Li Q, Zhang D, Wang L. Juglone exerts antitumor effect in ovarian cancer cells. Iran J Basic Med Sci. 2015; 18:544–548.13. Ahmad T, Suzuki YJ. Juglone in oxidative stress and cell signaling. Antioxidants (Basel). 2019; 8:E91.14. Rozentsvit A, Vinokur K, Samuel S, Li Y, Gerdes AM, Carrillo-Sepulveda MA. Ellagic acid reduces high glucose-induced vascular oxidative stress through ERK1/2/NOX4 signaling pathway. Cell Physiol Biochem. 2017; 44:1174–1187.15. Chen P, Chen F, Zhou B. Antioxidative, anti-inflammatory and anti-apoptotic effects of ellagic acid in liver and brain of rats treated by D-galactose. Sci Rep. 2018; 8:1465.16. Ji Y, Qu Z, Zou X, Ji C. Effects of juglone on ROS production and mitochondrial transmembrane potential in SGC-7901 cells. In : 2010 4th International Conference on Bioinformatics and Biomedical Engineering; 2010 Jun 18-20; Chengdu, China. Piscataway, NJ: Institute of Electrical and Electronics Engineers;2010.17. Costantino S, Paneni F, Lüscher TF, Cosentino F. Pin1 inhibitor Juglone prevents diabetic vascular dysfunction. Int J Cardiol. 2016; 203:702–707.18. Rodrigo R, Fernández-Gajardo R, Gutiérrez R, Matamala JM, Carrasco R, Miranda-Merchak A, Feuerhake W. Oxidative stress and pathophysiology of ischemic stroke: novel therapeutic opportunities. CNS Neurol Disord Drug Targets. 2013; 12:698–714.19. Lee JH, Kim H, Shim JH, Park J, Lee SK, Park KK, Chung WY. Platycarya strobilacea leaf extract inhibits tumor necrosis factor-αproduction and bone loss induced by Porphyromonas gingivalis-derived lipopolysaccharide. Arch Oral Biol. 2018; 96:46–51.20. Kim EJ, Choi JY, Park BC, Lee BH. Platycarya strobilacea S. et Z. Extract has a high antioxidant capacity and exhibits hair growth-promoting effects in male C57BL/6 mice. Prev Nutr Food Sci. 2014; 19:136–144.21. Zhang L, Wang Y, Xu M. In vitro antitumor activities of Platycarya strobilacea Sieb et Zucc infructescence extracts. Trop J Pharm Res. 2014; 13:849–854.22. Zhang LL, Wang YM, Xu M. Preparation and antimicrobial activity of tannin polymers from Platycarya strobilacea infructescence. Mater Res Innov. 2014; 18:S2-1046-S2-1049.23. El Hadrami A, Kone D, Lepoivre P. Effect of juglone on active oxygen species and antioxidant enzymes in susceptible and partially resistant banana cultivars to black leaf streak disease. Eur J Plant Pathol. 2005; 113:241–254.24. Han DH, Lee MJ, Kim JH. Antioxidant and apoptosis-inducing activities of ellagic acid. Anticancer Res. 2006; 26:3601–3606.25. National Research Council of the National Academies. Guide for the care and use of laboratory animals. 8th ed. Washington, DC: The National Academies Press;2011.26. Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989; 20:84–91.27. Takeshita H, Yamamoto K, Nozato S, Inagaki T, Tsuchimochi H, Shirai M, Yamamoto R, Imaizumi Y, Hongyo K, Yokoyama S, Takeda M, Oguro R, Takami Y, Itoh N, Takeya Y, Sugimoto K, Fukada SI, Rakugi H. Modified forelimb grip strength test detects aging-associated physiological decline in skeletal muscle function in male mice. Sci Rep. 2017; 7:42323.28. Drapeau E, Riad M, Kajiwara Y, Buxbaum JD. Behavioral phenotyping of an improved mouse model of Phelan-McDermid syndrome with a complete deletion of the shank3 gene. eNeuro. 2018; 5:ENEURO.0046-18.2018.29. Jeong JH, An JH, Yang H, Kim DK, Lee NS, Jeong YG, Na CS, Na DS, Dong MS, Han SY. Protective effect of Rhus verniciflua Stokes extract in an experimental model of post-menopausal osteoporosis. Anat Cell Biol. 2017; 50:219–229.30. Mir MA, Al-Baradie RS, Alhassainawi MD. Recent advances in stroke therapeutics. Hauppauge, NY: Nova Science Publishers;2014. p. 25–80.31. Duris K, Rolland WB, Zhang JH. Stroke pathophysiology and reactive oxygen species. In : Laher I, editor. Systems Biology of Free Radicals and Antioxidants. Berlin: Springer;2014. p. 1979–1997.32. Olmez I, Ozyurt H. Reactive oxygen species and ischemic cerebrovascular disease. Neurochem Int. 2012; 60:208–212.33. Tsai CC, Wu SB, Cheng CY, Kao SC, Kau HC, Chiou SH, Hsu WM, Wei YH. Increased oxidative DNA damage, lipid peroxidation, and reactive oxygen species in cultured orbital fibroblasts from patients with Graves' ophthalmopathy: evidence that oxidative stress has a role in this disorder. Eye (Lond). 2010; 24:1520–1525.34. Zhang H, Tsao R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr Opin Food Sci. 2016; 8:33–42.35. Zhang LL, Xu M, Wang YM, Wu DM, Chen JH. Optimizing ultrasonic ellagic acid extraction conditions from infructescence of Platycarya strobilacea using response surface methodology. Molecules. 2010; 15:7923–7932.36. Maoyi W, Jungtian L, Ning H. Determination of gallic acid in Platycarya strobilacea Sieb. et Zucc by RP-HPLC. China Pharm. 2010; 3:378–379.37. Nour V, Trandafir I, Cosmulescu S. HPLC determination of phenolic acids, flavonoids and juglone in walnut leaves. J Chromatogr Sci. 2013; 51:883–890.38. Altemimi A, Watson DG, Kinsel M, Lightfoot DA. Simultaneous extraction, optimization, and analysis of flavonoids and polyphenols from peach and pumpkin extracts using a TLC-densitometric method. Chem Cent J. 2015; 9:39.39. Bajpai VK, Alam MB, Quan KT, Kwon KR, Ju MK, Choi HJ, Lee JS, Yoon JI, Majumder R, Rather IA, Kim K, Lee SH, Na M. Antioxidant efficacy and the upregulation of Nrf2-mediated HO-1 expression by (+)-lariciresinol, a lignan isolated from Rubia philippinensis, through the activation of p38. Sci Rep. 2017; 7:46035.40. He M, Pan H, Chang RC, So KF, Brecha NC, Pu M. Activation of the Nrf2/HO-1 antioxidant pathway contributes to the protective effects of Lycium barbarum polysaccharides in the rodent retina after ischemia-reperfusion-induced damage. PLoS One. 2014; 9:e84800.41. Bereczki D Jr, Balla J, Bereczki D. Heme oxygenase-1: clinical relevance in ischemic stroke. Curr Pharm Des. 2018; 24:2229–2235.42. Lu X, Gu R, Hu W, Sun Z, Wang G, Wang L, Xu Y. Upregulation of heme oxygenase-1 protected against brain damage induced by transient cerebral ischemia-reperfusion injury in rats. Exp Ther Med. 2018; 15:4629–4636.43. Taguchi K, Soma O, Tsuneishi S, Takada S, Nakamura H. Role of heme oxygenase-1 (HO-1) in hypoxic-ischemic insult of the newborn rat brain. Pediatr Res. 1999; 45:228.44. Shah ZA, Nada SE, Doré S. Heme oxygenase 1, beneficial role in permanent ischemic stroke and in Gingko biloba (EGb 761) neuroprotection. Neuroscience. 2011; 180:248–255.45. Kansanen E, Kuosmanen SM, Leinonen H, Levonen AL. The Keap1-Nrf2 pathway: Mechanisms of activation and dysregulation in cancer. Redox Biol. 2013; 1:45–49.46. Loboda A, Damulewicz M, Pyza E, Jozkowicz A, Dulak J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell Mol Life Sci. 2016; 73:3221–3247.47. Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007; 47:89–116.48. Ye J, Piao H, Jiang J, Jin G, Zheng M, Yang J, Jin X, Sun T, Choi YH, Li L, Yan G. Polydatin inhibits mast cell-mediated allergic inflammation by targeting PI3K/Akt, MAPK, NF-κB and Nrf2/HO-1 pathways. Sci Rep. 2017; 7:11895.49. Kaulmann A, Bohn T. Bioactivity of polyphenols: preventive and adjuvant strategies toward reducing inflammatory bowel diseases-promises, perspectives, and pitfalls. Oxid Med Cell Longev. 2016; 2016:9346470.50. Scapagnini G, Vasto S, Abraham NG, Caruso C, Zella D, Fabio G. Modulation of Nrf2/ARE pathway by food polyphenols: a nutritional neuroprotective strategy for cognitive and neurodegenerative disorders. Mol Neurobiol. 2011; 44:192–201.51. Ding Y, Zhang B, Zhou K, Chen M, Wang M, Jia Y, Song Y, Li Y, Wen A. Dietary ellagic acid improves oxidant-induced endothelial dysfunction and atherosclerosis: role of Nrf2 activation. Int J Cardiol. 2014; 175:508–514.52. Badhani B, Sharma N, Kakkar R. Gallic acid: a versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 2015; 5:27540–27557.53. Kim SE, Lee MY, Lim SC, Hien TT, Kim JW, Ahn SG, Yoon JH, Kim SK, Choi HS, Kang KW. Role of Pin1 in neointima formation: down-regulation of Nrf2-dependent heme oxygenase-1 expression by Pin1. Free Radic Biol Med. 2010; 48:1644–1653.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Treatment with MnTBAP Protects Against Early Nuclear Translocation of Endonuclease G and Reduces Cerebral Infarction after Focal Cerebral Ischemia/Reperfusion in Mice
- A Study of Effect of Recirculation in Experimental Cerebral Ischemia
- Animal Model of Cerebral Ischemia
- Neuroprotective Effect of Focal Ischemic Preconditioning in Transient Focal Ischemia Animal Model
- Effect of Glutamate Antagonist upon Brain Edema in Long-Term Experimental Medel of Focal Cerebral Ischemia in Rats