World J Mens Health.
2020 Jan;38(1):68-77. 10.5534/wjmh.180052M.
Long-Term Testosterone Therapy in Type 2 Diabetes Is Associated with Decreasing Waist Circumference and Improving Erectile Function
- Affiliations
-
- 1Department of Urology, University Hospitals Birmingham NHS Foundation Trust, England, UK. hackettgeoff@gmail.com
- 2School of Health and Life Sciences, Aston University, England, UK.
- 3University of Cambridge, England, UK.
- 4Institute for Science and Technology in Medicine, Keele University Medical School, England, UK.
- 5Department of Clinical Biochemistry, University Hospitals Birmingham NHS Foundation Trust, England, UK.
- 6College of Engineering, Design and Physical Sciences, Brunel University London, England, UK.
- 7Department of Clinical Biochemistry, University Hospitals of North Midlands/Faculty of Health Sciences, Staffordshire University, England, UK.
- KMID: 2465411
- DOI: http://doi.org/10.5534/wjmh.180052M
Abstract
- PURPOSE
To describe the 4-year metabolic follow-up results from the BLAST study.
MATERIALS AND METHODS
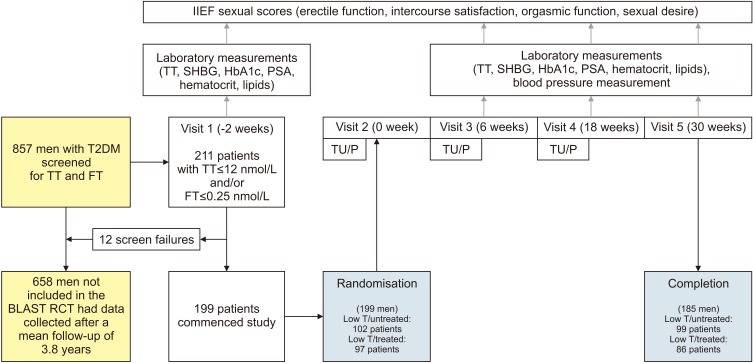
Baseline hemoglobin A1c (HbA1c), weight, and waist circumference (WC) data were recorded in 185 men recruited for the BLAST randomised controlled trial (RCT) and erectile function (EF) scores were also available in an additional 48 men screened for the RCT. Intra/inter-group associations between these parameters and testosterone replacement therapy (TRT) were assessed at 1) end of the RCT (30 weeks), 2) open-label phase (82 weeks), and 3) final assessment via non-parametric statistics.
RESULTS
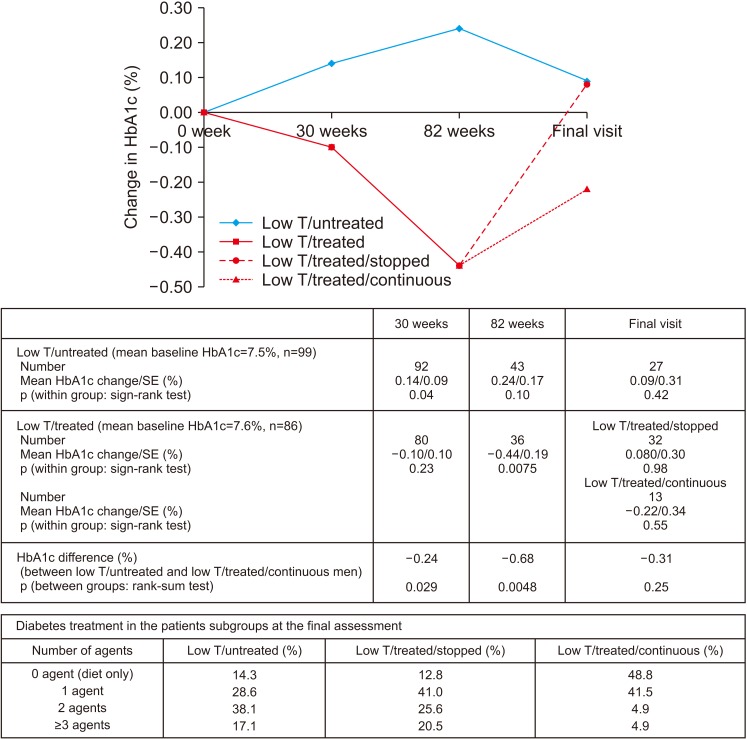
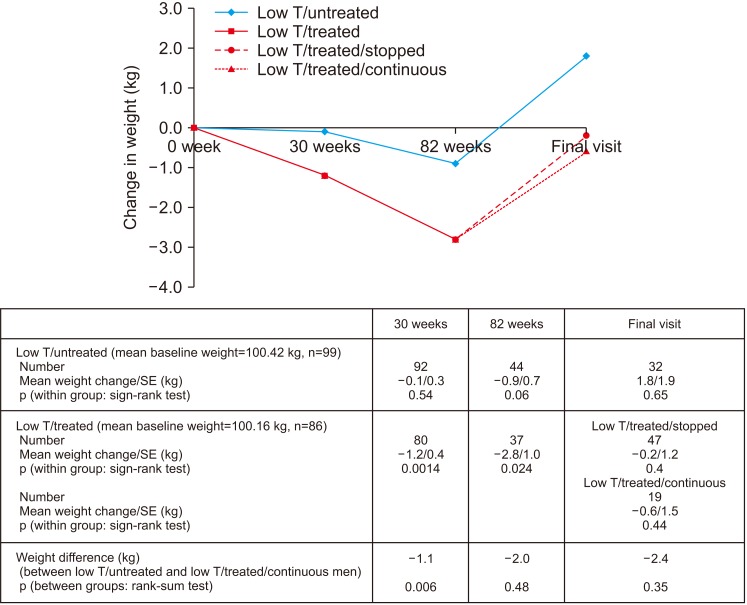
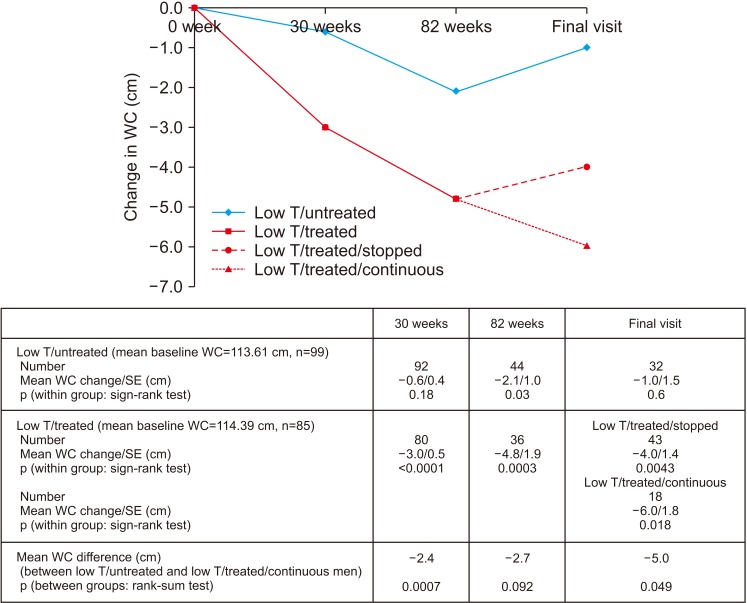
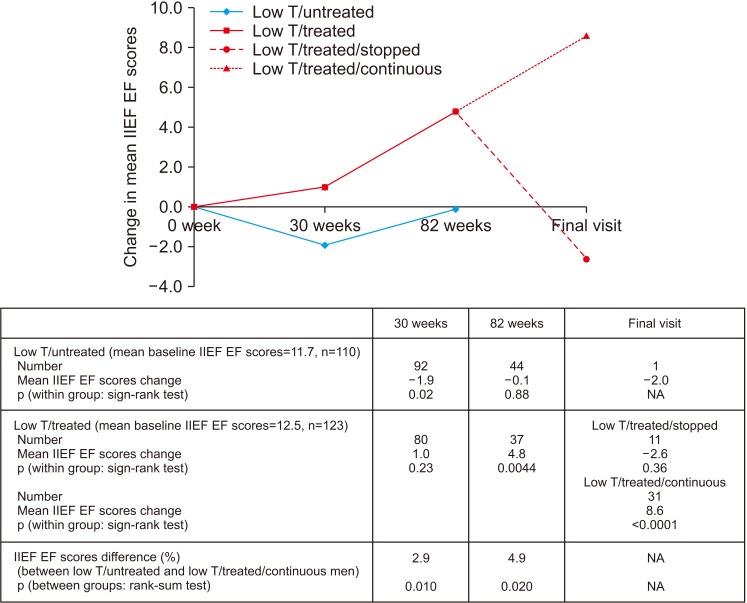
Improvement in HbA1c and weight at the end of the RCT and open-label phase in men on TRT was not maintained long-term. The convergence in HbA1c could have been due to incentivised care with HbA1c targets. Interestingly those on TRT at final assessment required fewer anti-diabetic agents. The weight increase in routine care may have been due to changes in diabetes medication or an increase in lean muscle mass. WC continued to decrease in men on TRT indicating possible reduction in visceral fat. Improvement in EF scores continued with long-term TRT, this was abolished when TRT was discontinued.
CONCLUSIONS
This study hints at benefits in glycaemic control, weight and WC, and long-term RCTs studying mechanisms of benefit and clinical outcomes are necessary. Our results also show that EF scores continued to improve with long-term TRT, even beyond the 6 months that we previously reported in the BLAST RCT.
MeSH Terms
Figure
Reference
-
1. Hackett G, Cole N, Deshpande A, Popple M, Kennedy D, Wilkinson P. Biochemical hypodonadism and type 2 diabetes in primary care. Br J Diab Vasc Dis. 2009; 9:226–231.2. Saad F, Haider A, Doros G, Traish A. Long-term treatment of hypogonadal men with testosterone produces substantial and sustained weight loss. Obesity (Silver Spring). 2013; 21:1975–1981. PMID: 23512691.
Article3. Traish AM, Haider A, Doros G, Saad F. Long-term testosterone therapy in hypogonadal men ameliorates elements of the metabolic syndrome: an observational, long-term registry study. Int J Clin Pract. 2014; 68:314–329. PMID: 24127736.
Article4. Hackett G, Cole N, Bhartia M, Kennedy D, Raju J, Wilkinson P, et al. The response to testosterone undecanoate in men with type 2 diabetes is dependent on achieving threshold serum levels (the BLAST study). Int J Clin Pract. 2014; 68:203–215. PMID: 24355040.
Article5. Hackett G, Cole N, Bhartia M, Kennedy D, Raju J, Wilkinson P. Testosterone replacement therapy improves metabolic parameters in hypogonadal men with type 2 diabetes but not in men with coexisting depression: the BLAST study. J Sex Med. 2014; 11:840–856. PMID: 24308723.
Article6. Hackett G, Cole N, Saghir A, Jones P, Strange RC, Ramachandran S. Testosterone undecanoate improves sexual function in men with type 2 diabetes and severe hypogonadism: results from a 30-week randomized placebo-controlled study. BJU Int. 2016; 118:804–813. PMID: 27124889.
Article7. Hackett G, Cole N, Saghir A, Jones P, Strange RC, Ramachandran S. Testosterone replacement therapy: improved sexual desire and erectile function in men with type 2 diabetes following a 30-week randomized placebo-controlled study. Andrology. 2017; 5:905–913. PMID: 28771964.
Article8. Hackett G, Heald AH, Sinclair A, Jones PW, Strange RC, Ramachandran S. Serum testosterone, testosterone replacement therapy and all-cause mortality in men with type 2 diabetes: retrospective consideration of the impact of PDE5 inhibitors and statins. Int J Clin Pract. 2016; 70:244–253. PMID: 26916621.
Article9. Hackett G, Jones PW, Strange RC, Ramachandran S. Statin, testosterone and phosphodiesterase 5-inhibitor treatments and age related mortality in diabetes. World J Diabetes. 2017; 8:104–111. PMID: 28344753.
Article10. Dohle GR, Arver S, Bettocchi C, Kliesch S, Punab M, de Ronde W. Guidelines on Male Hypogonadism [Internet]. Arnhem: European Association of Urology;c2014. cited 2012 Mar 6. Available from: https://uroweb.org/wp-content/uploads/18-Male-Hypogonadism_LR.pdf.11. Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999; 84:3666–3672. PMID: 10523012.
Article12. Grossmann M, Hoermann R, Wittert G, Yeap BB. Effects of testosterone treatment on glucose metabolism and symptoms in men with type 2 diabetes and the metabolic syndrome: a systematic review and meta-analysis of randomized controlled clinical trials. Clin Endocrinol (Oxf). 2015; 83:344–351. PMID: 25557752.
Article13. Corona G, Giagulli VA, Maseroli E, Vignozzi L, Aversa A, Zitzmann M, et al. Therapy of endocrine disease: testosterone supplementation and body composition: results from a meta-analysis study. Eur J Endocrinol. 2016; 174:R99–R116. PMID: 26537862.
Article14. Ng Tang Fui M, Prendergast LA, Dupuis P, Raval M, Strauss BJ, Zajac JD, et al. Effects of testosterone treatment on body fat and lean mass in obese men on a hypocaloric diet: a randomised controlled trial. BMC Med. 2016; 14:153. PMID: 27716209.
Article15. Bhasin S, Brito JP, Cunningham GR, Hayes FJ, Hodis HN, Matsumoto AM, et al. Testosterone therapy in men with hypogonadism: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2018; 103:1715–1744. PMID: 29562364.
Article16. Corona G, Maseroli E, Rastrelli G, Isidori AM, Sforza A, Mannucci E, et al. Cardiovascular risk associated with testosterone-boosting medications: a systematic review and meta-analysis. Expert Opin Drug Saf. 2014; 13:1327–1351. PMID: 25139126.
Article17. Corona G, Rastrelli G, Morgentaler A, Sforza A, Mannucci E, Maggi M. Meta-analysis of results of testosterone therapy on sexual function based on international index of erectile function scores. Eur Urol. 2017; 72:1000–1011. PMID: 28434676.
Article18. Borst SE, Yarrow JF. Injection of testosterone may be safer and more effective than transdermal administration for combating loss of muscle and bone in older men. Am J Physiol Endocrinol Metab. 2015; 308:E1035–E1042. PMID: 25898953.
Article19. McEwan P, Bennett H, Qin L, Bergenheim K, Gordon J, Evans M. An alternative approach to modelling HbA1c trajectories in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2017; 19:628–634. PMID: 28026908.
Article20. DHSC 2003. Investing in General Practice: Implementing the New GMS Contract [Internet]. London: Department of Health Social Care;cited 2018 May 5. Available from: http://www.nhsemployers.org/~/media/Employers/Documents/SiteCollectionDocuments/gms_contract_cd_130209.pdf.21. Clarke EL, Richardson JR, Bhartia M, Kennedy DM, Milles JJ, Ramachandran S. Convergence of HbA1c values towards target in 272 primary care patients following nine years of target-driven care. Qual Prim Care. 2013; 21:287–292. PMID: 24119514.22. Srinivas-Shankar U, Roberts SA, Connolly MJ, O'Connell MD, Adams JE, Oldham JA, et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2010; 95:639–650. PMID: 20061435.
Article23. Saad F, Röhrig G, von Haehling S, Traish A. Testosterone deficiency and testosterone treatment in older men. Gerontology. 2017; 63:144–156. PMID: 27855417.
Article24. Garvey WT, Mechanick JI, Brett EM, Garber AJ, Hurley DL, Jastreboff AM, et al. Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines. AACE/ACE guidelines: American Association of Clinical Endocrinologists and American College of Endocrinology clinical practice guidelines for comprehensive medical care of patients with obesity. Endocr Pract. 2016; 22:842–884. PMID: 27472012.25. Mulhall JP, Trost LW, Brannigan RE, Kurtz EG, Redmon JB, Chiles KA, et al. Evaluation and management of testosterone deficiency: AUA guideline. J Urol. 2018; 200:423–432. PMID: 29601923.
Article26. Shipman KE, Strange RC, Ramachandran S. Use of fibrates in the metabolic syndrome: a review. World J Diabetes. 2016; 7:74–88. PMID: 26981181.
Article27. Snyder PJ, Bhasin S, Cunningham GR, Matsumoto AM, Stephens-Shields AJ, Cauley JA, et al. Effects of testosterone treatment in older men. N Engl J Med. 2016; 374:611–624. PMID: 26886521.
Article28. Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010; 95:2536–2559. PMID: 20525905.
Article29. Ponce OJ, Spencer-Bonilla G, Alvarez-Villalobos N, Serrano V, Singh-Ospina N, Rodriguez-Gutierrez R, et al. The efficacy and adverse events of testosterone replacement therapy in hypogonadal men: a systematic review and meta-analysis of randomized, placebo-controlled trials. J Clin Endocrinol Metab. 2018; 103:1745–1754.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Testosterone Replacement Treatment in Testosterone Deficiency Syndrome Patients with Metabolic Syndrome
- The Efficacy of a Combination of Phosphodiesterase Type 5 Inhibitor and Testosterone Replacement Therapy in Nonresponders to Phosphodiesterase Type 5 Inhibitor Mono-therapy
- Correlation of the Serum Testosterone Level with Insulin Resistance and Metabolic Syndrome in Patients of Erectile Dysfunction and Benign Prostatic Hyperplasia
- The Effect of Andriol on the Sexual Function in the Aging Male
- Factors Associated with Serum Testosterone Levels in Obese Men Aged 20–39 Years