Nutr Res Pract.
2019 Dec;13(6):480-487. 10.4162/nrp.2019.13.6.480.
Study of the cartilage matrix production-promoting effect of chicken leg extract and identification of the active ingredient
- Affiliations
-
- 1Pharma Foods International Co., Ltd., 1-49 Goryo-Ohara, Nishikyo-ku, Kyoto 615-8245, Japan. a-yamatsu@pharmafoods.co.jp
- KMID: 2464127
- DOI: http://doi.org/10.4162/nrp.2019.13.6.480
Abstract
- BACKGROUND/OBJECTIVES
Osteoarthritis (OA) is a major public health issue in Japan and other countries, and foods that prevent or treat OA are in strong demand. Proteins and peptides in chicken meat and bones are known for being rich in functional and nutritional ingredients for the improvement of osteoporosis. We speculated that chicken legs, a food consumed in many regions of the world, may also contain such ingredients. In this study, we aim to (i) evaluate the effect of chicken leg extract (CLE) on the promotion of cartilage matrix production and (ii) identify the active ingredient in CLE that contributes to this function.
MATERIALS/METHODS
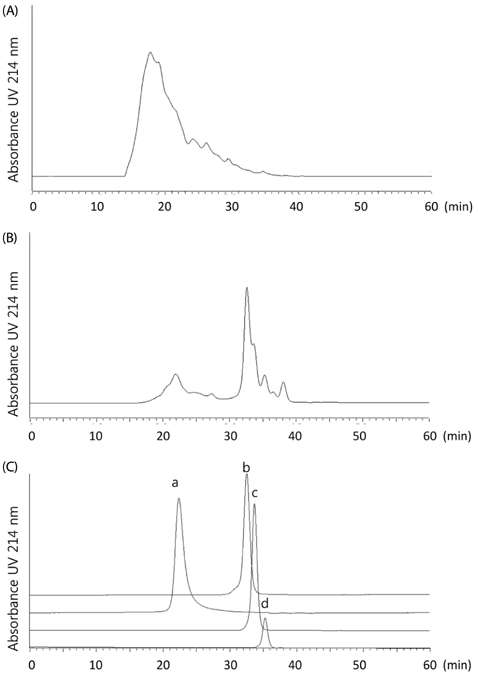
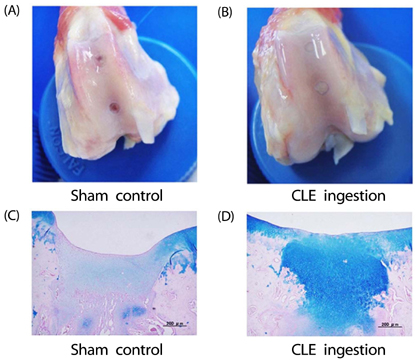
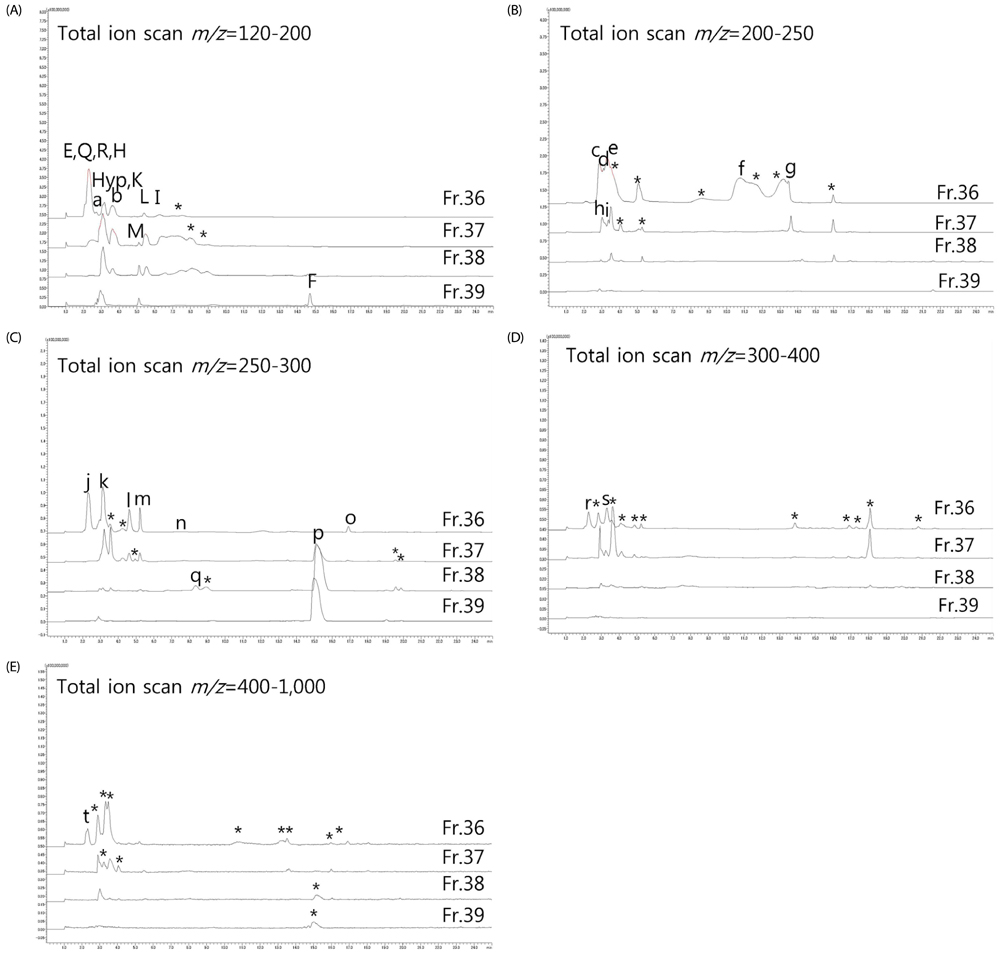
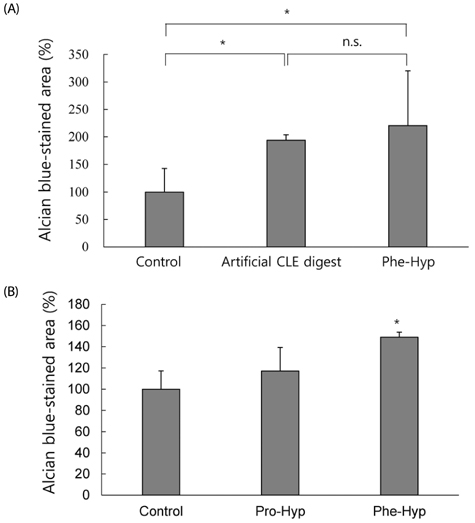
Artificial CLE digest was prepared, and the acid mucopolysaccharide production-promoting activity of the CLE digest was evaluated by alcian blue staining of ATDC5 cells. CLE was orally administered to rabbits with burr holes in the knee joint of the femur, and the degree of regeneration of cartilage matrix was evaluated. Furthermore, we investigated orally administered CLE-derived peptides in human plasma using LC-MS. From measuring the acid mucopolysaccharide production-promotion activity of these peptides, a molecule considered to be an active ingredient in the CLE digest was identified.
RESULTS
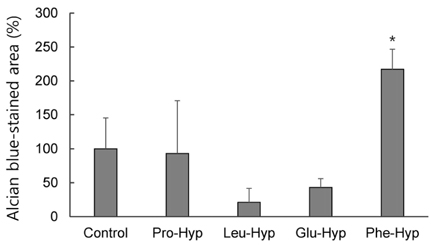
CLE digest promoted acid mucopolysaccharide production and facilitated regeneration of cartilage matrix in in vitro and in vivo experiments. Four peptides including phenylalanyl-hydroxyproline (Phe-Hyp) were detected as CLE-derived peptides in human plasma. The effect of CLE was inferred to be due to Phe-Hyp, which was confirmed to be present in the CLE digest.
CONCLUSIONS
It was shown that CLE stimulated the production of articular cartilage matrix both in vitro and in vivo, and that CLE could be an effective food for preventing or treating OA. Furthermore, only Phe-Hyp was confirmed as the active compound in the CLE digest, suggesting that the activity of CLE was due to Phe-Hyp.
MeSH Terms
Figure
Reference
-
1. Dreier R. Hypertrophic differentiation of chondrocytes in osteoarthritis: the developmental aspect of degenerative joint disorders. Arthritis Res Ther. 2010; 12:216.
Article2. Toda Y, Toda T, Takemura S, Wada T, Morimoto T, Ogawa R. Change in body fat, but not body weight or metabolic correlates of obesity, is related to symptomatic relief of obese patients with knee osteoarthritis after a weight control program. J Rheumatol. 1998; 25:2181–2186.3. Dominick KL, Ahern FM, Gold CH, Heller DA. Health-related quality of life among older adults with arthritis. Health Qual Life Outcomes. 2004; 2:5.4. Yoshimura N, Muraki S, Oka H, Mabuchi A, En-Yo Y, Yoshida M, Saika A, Yoshida H, Suzuki T, Yamamoto S, Ishibashi H, Kawaguchi H, Nakamura K, Akune T. Prevalence of knee osteoarthritis, lumbar spondylosis, and osteoporosis in Japanese men and women: the research on osteoarthritis/osteoporosis against disability study. J Bone Miner Metab. 2009; 27:620–628.
Article5. Silverstein FE, Faich G, Goldstein JL, Simon LS, Pincus T, Whelton A, Makuch R, Eisen G, Agrawal NM, Stenson WF, Burr AM, Zhao WW, Kent JD, Lefkowith JB, Verburg KM, Geis GS. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: a randomized controlled trial. Celecoxib long-term arthritis safety study. JAMA. 2000; 284:1247–1255.
Article6. Rönn K, Reischl N, Gautier E, Jacobi M. Current surgical treatment of knee osteoarthritis. Arthritis. 2011; 2011:454873.
Article7. Bitton R. The economic burden of osteoarthritis. Am J Manag Care. 2009; 15:S230–S235.8. Gregory PJ, Fellner C. Dietary supplements as disease-modifying treatments in osteoarthritis: a critical appraisal. P T. 2014; 39:436–452.9. Schonfeldt HC, Pretorius B, Hall N. “Fish, chicken, lean meat and eggs can be eaten daily”: food-based dietary guidelines for South Africa. South Afr J Clin Nutr. 2013; 26:S66–S76.10. Paddon-Jones D, Rasmussen BB. Dietary protein recommendations and the prevention of sarcopenia. Curr Opin Clin Nutr Metab Care. 2009; 12:86–90.
Article11. Kawamura S, Wakitani S, Kimura T, Maeda A, Caplan AI, Shino K, Ochi T. Articular cartilage repair. Rabbit experiments with a collagen gel-biomatrix and chondrocytes cultured in it. Acta Orthop Scand. 1998; 69:56–62.
Article12. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012; 9:671–675.
Article13. Abramoff MD, Magelhaes PJ, Ram SJ. Image processing with ImageJ. Biophoton Int. 2004; 11:36–42.14. Sugihara F, Inoue N, Kuwamori M, Taniguchi M. Quantification of hydroxyprolyl-glycine (Hyp-Gly) in human blood after ingestion of collagen hydrolysate. J Biosci Bioeng. 2012; 113:202–203.
Article15. Carpino LA. 1-Hydroxy-7-azabenzotriazole. An efficient peptide coupling additive. J Am Chem Soc. 1993; 115:4397–4398.
Article16. Han G, Tamaki M, Hruby VJ. Fast, efficient and selective deprotection of the tert-butoxycarbonyl (Boc) group using HCl/dioxane (4 m). J Pept Res. 2001; 58:338–341.
Article17. Vermeirssen V, Van Camp J, Devos L, Verstraete W. Release of angiotensin I converting enzyme (ACE) inhibitory activity during in vitro gastrointestinal digestion: from batch experiment to semicontinuous model. J Agric Food Chem. 2003; 51:5680–5687.
Article18. Hernández-Ledesma B, Quirós A, Amigo L, Recio I. Identification of bioactive peptides after digestion of human milk and infant formula with pepsin and pancreatin. Int Dairy J. 2007; 17:42–49.
Article19. Matoba T, Doi E, Yonezawa D. Digestibility of acetylated and succinylated proteins by pepsin-pancreatin and some intracellular peptidases. Agric Biol Chem. 1980; 44:2323–2328.
Article20. Culling CF. Carbohydrate. In : Culling CFA, editor. Handbook of Histopathological and Histochemical Technique. . London: Butterworths-Heinemann;1974. p. 275–290.21. Atsumi T, Miwa Y, Kimata K, Ikawa Y. A chondrogenic cell line derived from a differentiating culture of AT805 teratocarcinoma cells. Cell Differ Dev. 1990; 30:109–116.
Article22. Yamamoto K, Santamaria S, Botkjaer KA, Dudhia J, Troeberg L, Itoh Y, Murphy G, Nagase H. Inhibition of shedding of low-density lipoprotein receptor-related protein 1 reverses cartilage matrix degradation in osteoarthritis. Arthritis Rheumatol. 2017; 69:1246–1256.
Article23. Pearle AD, Warren RF, Rodeo SA. Basic science of articular cartilage and osteoarthritis. Clin Sports Med. 2005; 24:1–12.
Article24. Liu C, Sugita K, Nihei K, Yoneyama K, Tanaka H. Absorption of hydroxyproline-containing peptides in vascularly perfused rat small intestine in situ. Biosci Biotechnol Biochem. 2009; 73:1741–1747.
Article25. Nakatani S, Mano H, Sampei C, Shimizu J, Wada M. Chondroprotective effect of the bioactive peptide prolyl-hydroxyproline in mouse articular cartilage in vitro and in vivo. Osteoarthritis Cartilage. 2009; 17:1620–1627.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anti-inflammatory effects of ethanolic extract of Annona muricata
- Expression of Chicken Cartilage Derived Matrix Protein 10 (CCMP 10) in Chondrogenesis
- Cedrol Enhances Extracellular Matrix Production in Dermal Fibroblasts in a MAPK-Dependent Manner
- Giant Cell Tumor with an Unusual Cartilage Matrix: A Case Report
- The effect of leg lenghening on the articular cartilage of the rabbit tibia