J Korean Neurosurg Soc.
2019 Jul;62(4):442-449. 10.3340/jkns.2018.0178.
Machine Learning Model to Predict Osteoporotic Spine with Hounsfield Units on Lumbar Computed Tomography
- Affiliations
-
- 1Department of Neurosurgery, Medical Research Institute, Pusan National University Hospital, Busan, Korea. farlateral@hanmail.net
- KMID: 2463675
- DOI: http://doi.org/10.3340/jkns.2018.0178
Abstract
OBJECTIVE
Bone mineral density (BMD) is an important consideration during fusion surgery. Although dual X-ray absorptiometry is considered as the gold standard for assessing BMD, quantitative computed tomography (QCT) provides more accurate data in spine osteoporosis. However, QCT has the disadvantage of additional radiation hazard and cost. The present study was to demonstrate the utility of artificial intelligence and machine learning algorithm for assessing osteoporosis using Hounsfield units (HU) of preoperative lumbar CT coupling with data of QCT.
METHODS
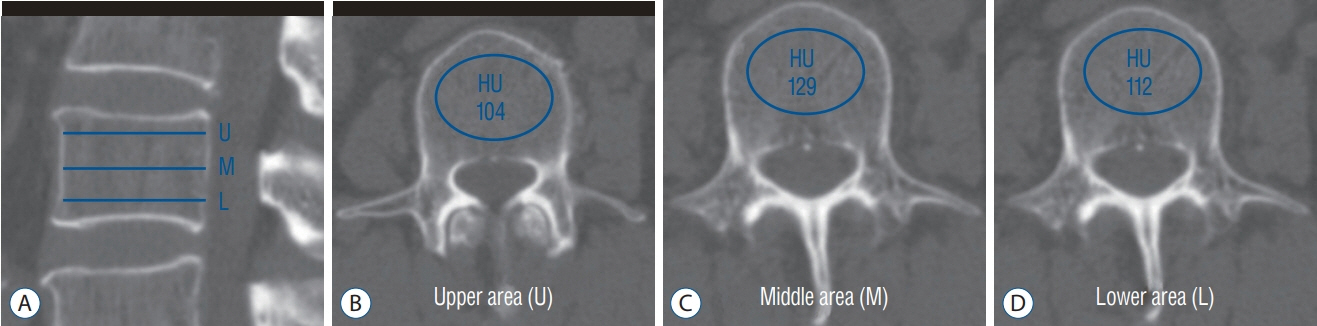
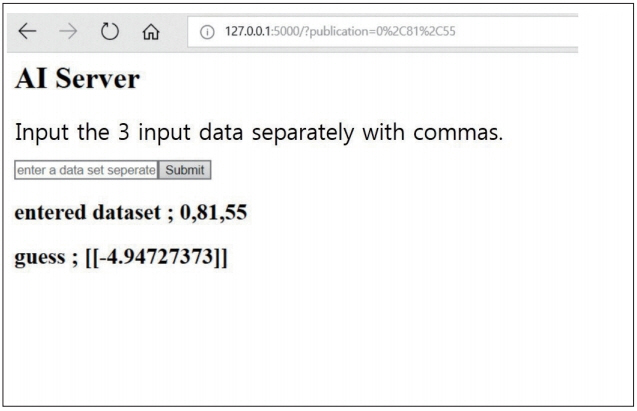
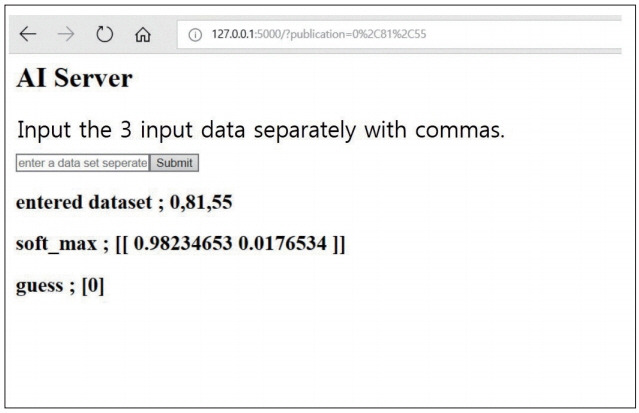
We reviewed 70 patients undergoing both QCT and conventional lumbar CT for spine surgery. The T-scores of 198 lumbar vertebra was assessed in QCT and the HU of vertebral body at the same level were measured in conventional CT by the picture archiving and communication system (PACS) system. A multiple regression algorithm was applied to predict the T-score using three independent variables (age, sex, and HU of vertebral body on conventional CT) coupling with T-score of QCT. Next, a logistic regression algorithm was applied to predict osteoporotic or non-osteoporotic vertebra. The Tensor flow and Python were used as the machine learning tools. The Tensor flow user interface developed in our institute was used for easy code generation.
RESULTS
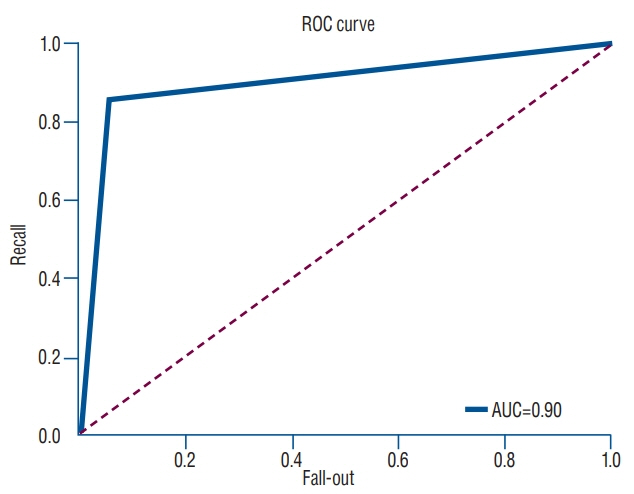
The predictive model with multiple regression algorithm estimated similar T-scores with data of QCT. HU demonstrates the similar results as QCT without the discordance in only one non-osteoporotic vertebra that indicated osteoporosis. From the training set, the predictive model classified the lumbar vertebra into two groups (osteoporotic vs. non-osteoporotic spine) with 88.0% accuracy. In a test set of 40 vertebrae, classification accuracy was 92.5% when the learning rate was 0.0001 (precision, 0.939; recall, 0.969; F1 score, 0.954; area under the curve, 0.900).
CONCLUSION
This study is a simple machine learning model applicable in the spine research field. The machine learning model can predict the T-score and osteoporotic vertebrae solely by measuring the HU of conventional CT, and this would help spine surgeons not to under-estimate the osteoporotic spine preoperatively. If applied to a bigger data set, we believe the predictive accuracy of our model will further increase. We propose that machine learning is an important modality of the medical research field.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Applications of Machine Learning in Bone and Mineral Research
Sung Hye Kong, Chan Soo Shin
Endocrinol Metab. 2021;36(5):928-937. doi: 10.3803/EnM.2021.1111.Neurosurgical Management of Cerebrospinal Tumors in the Era of Artificial Intelligence : A Scoping Review
Kuchalambal Agadi, Asimina Dominari, Sameer Saleem Tebha, Asma Mohammadi, Samina Zahid
J Korean Neurosurg Soc. 2023;66(6):632-641. doi: 10.3340/jkns.2021.0213.
Reference
-
References
1. Bastanlar Y, Ozuysal M. Introduction to machine learning. Methods Mol Biol. 1107:105–128. 2014.2. Cheng Q, Zhu YX, Zhang MX, Li LH, Du PY, Zhu MH. Age and sex effects on the association between body composition and bone mineral density in healthy Chinese men and women. Menopause. 19:448–455. 2012.
Article3. Choi MK, Kim SM, Lim JK. Diagnostic efficacy of Hounsfield units in spine CT for the assessment of real bone mineral density of degenerative spine: correlation study between T-scores determined by DEXA scan and Hounsfield units from CT. Acta Neurochir (Wien). 158:1421–1427. 2016.
Article4. Coe JD, Warden KE, Herzig MA, McAfee PC. Influence of bone mineral density on the fixation of thoracolumbar implants. A comparative study of transpedicular screws, laminar hooks, and spinous process wires. Spine (Phila Pa 1976). 15:902–907. 1990.
Article5. Deo RC. Machine learning in medicine. Circulation. 132:1920–1930. 2015.
Article6. Ebbesen EN, Thomsen JS, Beck-Nielsen H, Nepper-Rasmussen HJ, Mosekilde L. Lumbar vertebral body compressive strength evaluated by dual-energy X-ray absorptiometry, quantitative computed tomography, and ashing. Bone. 25:713–724. 1999.
Article7. Erickson BJ, Korfiatis P, Akkus Z, Kline TL. Machine learning for medical imaging. Radiographics. 37:505–515. 2017.
Article8. Forsting M. Machine learning will change medicine. J Nucl Med. 58:357–358. 2017.
Article9. Halvorson TL, Kelley LA, Thomas KA, Whitecloud TS 3rd, Cook SD. Effects of bone mineral density on pedicle screw fixation. Spine (Phila Pa 1976). 19:2415–2420. 1994.
Article10. Hu SS. Internal fixation in the osteoporotic spine. Spine (Phila Pa 1976). 22(24 Suppl):43S–48S. 1997.
Article11. Jergas M, Breitenseher M, Glüer CC, Black D, Lang P, Grampp S, et al. Which vertebrae should be assessed using lateral dual-energy X-ray absorptiometry of the lumbar spine. Osteoporos Int. 5:196–204. 1995.
Article12. Kotoku J. An introduction to machine learning. Igaku Butsuri. 36:18–22. 2016.13. Lee S, Chung CK, Oh SH, Park SB. Correlation between bone mineral density measured by dual-energy X-ray absorptiometry and Hounsfield units measured by diagnostic CT in lumbar spine. J Korean Neurosurg Soc. 54:384–389. 2013.
Article14. Lochmüller EM, Bürklein D, Kuhn V, Glaser C, Müller R, Glüer CC, et al. Mechanical strength of the thoracolumbar spine in the elderly: prediction from in situ dual-energy X-ray absorptiometry, quantitative computed tomography (QCT), upper and lower limb peripheral QCT, and quantitative ultrasound. Bone. 31:77–84. 2002.
Article15. Masud T, Langley S, Wiltshire P, Doyle DV, Spector TD. Effect of spinal osteophytosis on bone mineral density measurements in vertebral osteoporosis. BMJ. 307:172–173. 1993.
Article16. Matsukawa K, Abe Y, Yanai Y, Yato Y. Regional Hounsfield unit measurement of screw trajectory for predicting pedicle screw fixation using cortical bone trajectory: a retrospective cohort study. Acta Neurochir (Wien). 160:405–411. 2018.
Article17. Mounach A, Abayi DA, Ghazi M, Ghozlani I, Nouijai A, Achemlal L, et al. Discordance between hip and spine bone mineral density measurement using DXA: prevalence and risk factors. Semin Arthritis Rheum. 38:467–471. 2009.
Article18. Nguyen ND, Eisman JA, Center JR, Nguyen TV. Risk factors for fracture in nonosteoporotic men and women. J Clin Endocrinol Metab. 92:955–962. 2007.
Article19. Nonaka K, Uchiyama S. Assessment of volumetric bone mineral density and geometry for hip with clinical CT device. Clin Calcium. 21:1003–1009. 2011.20. Reynolds RJ, Day SM. The growing role of machine learning and artificial intelligence in developmental medicine. Dev Med Child Neurol. 60:858–859. 2018.
Article21. Schreiber JJ, Anderson PA, Rosas HG, Buchholz AL, Au AG. Hounsfield units for assessing bone mineral density and strength: a tool for osteoporosis management. J Bone Joint Surg Am. 93:1057–1063. 2011.
Article22. Somashekhar SP, Sepúlveda MJ, Puglielli S, Norden AD, Shortliffe EH, Rohit Kumar C, et al. Watson for oncology and breast cancer treatment recommendations: agreement with an expert multidisciplinary tumor board. Ann Oncol. 29:418–423. 2018.
Article23. Suzuki K. Pixel-based machine learning in medical imaging. Int J Biomed Imaging. 2012:792079. 2012.
Article24. Suzuki K, Yan P, Wang F, Shen D. Machine learning in medical imaging. Int J Biomed Imaging. 2012:123727. 2012.
Article25. Yamagata M, Kitahara H, Minami S, Takahashi K, Isobe K, Moriya H, et al. Mechanical stability of the pedicle screw fixation systems for the lumbar spine. Spine (Phila Pa 1976). 17(3 Suppl):S51–S54. 1992.
Article26. Younes M, Ben Hammouda S, Jguirim M, Younes K, Zrour S, Béjia I, et al. Discordance between spine and hip bone mineral density measurement using DXA in osteoporosis diagnosis: prevalence and risk factors. Tunis Med. 92:1–5. 2014.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Application of Machine Learning Algorithms to Predict Osteoporotic Fractures in Women
- Study for hounsfield units in computed tomogram with jaw lesion
- Applications of Machine Learning Using Electronic Medical Records in Spine Surgery
- Vitamin D levels and bone mineral density: a prospective cross-sectional analysis of young orthopedic trauma patients at a rural United States trauma center
- Machine Learning vs. Statistical Model for Prediction Modelling: Application in Medical Imaging Research