J Korean Neurosurg Soc.
2019 Jul;62(4):367-375. 10.3340/jkns.2018.0218.
Intraoperative Neurophysiological Monitoring during Microvascular Decompression Surgery for Hemifacial Spasm
- Affiliations
-
- 1Department of Neurology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 2Department of Neurology, Myongji Hospital, Hanyang University Medical Center, Goyang, Korea.
- 3Department of Neurosurgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. kwanmd.park@samsung.com
- KMID: 2463666
- DOI: http://doi.org/10.3340/jkns.2018.0218
Abstract
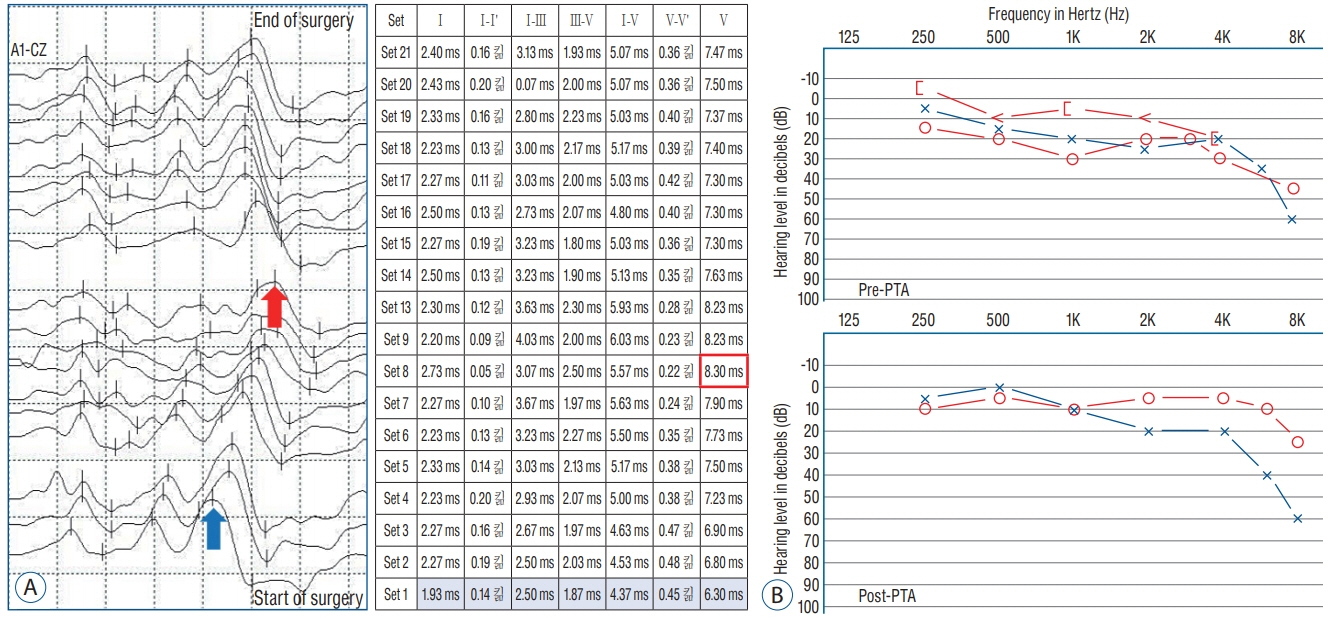
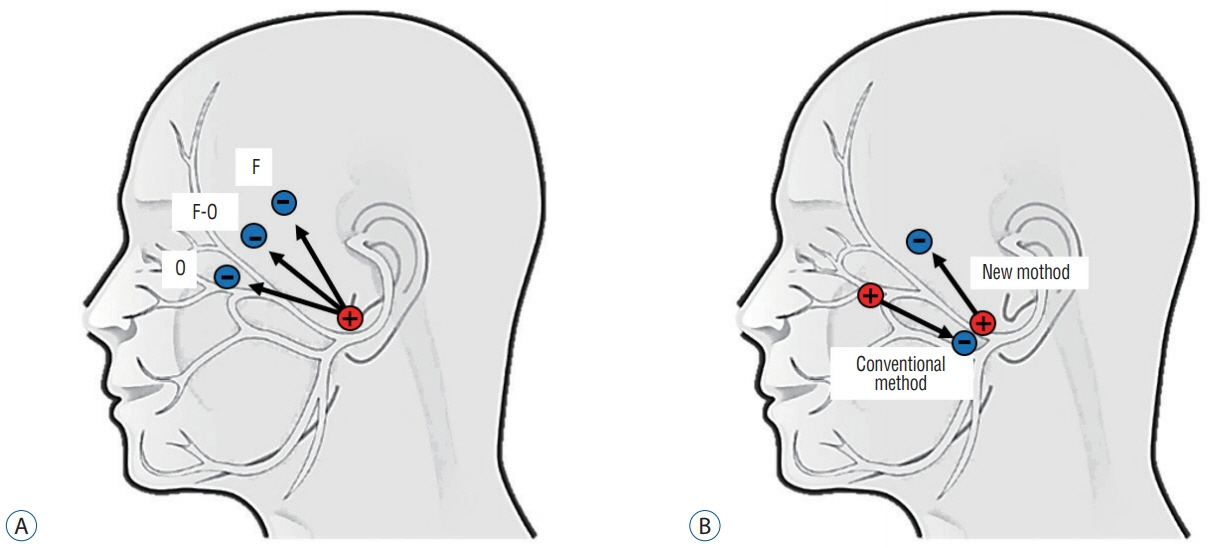
- Hemifacial spasm (HFS) is due to the vascular compression of the facial nerve at its root exit zone (REZ). Microvascular decompression (MVD) of the facial nerve near the REZ is an effective treatment for HFS. In MVD for HFS, intraoperative neurophysiological monitoring (INM) has two purposes. The first purpose is to prevent injury to neural structures such as the vestibulocochlear nerve and facial nerve during MVD surgery, which is possible through INM of brainstem auditory evoked potential and facial nerve electromyography (EMG). The second purpose is the unique feature of MVD for HFS, which is to assess and optimize the effectiveness of the vascular decompression. The purpose is achieved mainly through monitoring of abnormal facial nerve EMG that is called as lateral spread response (LSR) and is also partially possible through Z-L response, facial F-wave, and facial motor evoked potentials. Based on the information regarding INM mentioned above, MVD for HFS can be considered as a more safe and effective treatment.
Keyword
MeSH Terms
Figure
Reference
-
References
1. American Clinical Neurophysiology Society. Guideline 9C: guidelines on short-latency auditory evoked potentials. J Clin Neurophysiol. 23:157–167. 2006.2. American Electroencephalographic Society. Guideline eleven: guidelines for intraoperative monitoring of sensory evoked potentials. J Clin Neurophysiol. 11:77–87. 1994.3. Barker FG 2nd, Jannetta PJ, Bissonette DJ, Shields PT, Larkins MV, Jho HD. Microvascular decompression for hemifacial spasm. J Neurosurg. 82:201–210. 1995.
Article4. Chung SS, Chang JW, Kim SH, Chang JH, Park YG, Kim DI. Microvascular decompression of the facial nerve for the treatment of hemifacial spasm: preoperative magnetic resonance imaging related to clinical outcomes. Acta Neurochir (Wien). 142:901–906. discussion 907. 2000.
Article5. Fernández-Conejero I, Ulkatan S, Sen C, Deletis V. Intra-operative neurophysiology during microvascular decompression for hemifacial spasm. Clin Neurophysiol. 123:78–83. 2012.
Article6. Fukuda M, Takao T, Hiraishi T, Fujii Y. Free-running EMG monitoring during microvascular decompression for hemifacial spasm. Acta Neurochir (Wien). 157:1505–1512. 2015.
Article7. Grundy BL, Jannetta PJ, Procopio PT, Lina A, Boston JR, Doyle E. Intraoperative monitoring of brain-stem auditory evoked potentials. J Neurosurg. 57:674–681. 1982.
Article8. Haines SJ, Torres F. Intraoperative monitoring of the facial nerve during decompressive surgery for hemifacial spasm. J Neurosurg. 74:254–257. 1991.
Article9. Hatayama T, Møller AR. Correlation between latency and amplitude of peak V in the brainstem auditory evoked potentials: intraoperative recordings in microvascular decompression operations. Acta Neurochir (Wien). 140:681–687. 1998.
Article10. Hatem J, Sindou M, Vial C. Intraoperative monitoring of facial EMG responses during microvascular decompression for hemifacial spasm. Prognostic value for long-term outcome: a study in a 33-patient series. Br J Neurosurg. 15:496–499. 2001.
Article11. Hyun SJ, Kong DS, Park K. Microvascular decompression for treating hemifacial spasm: lesion learned from a prospective study of 1,174 operations. Neurosurg Rev. 33:325–334. 2010.
Article12. Ishikawa M, Ohira T, Namiki J, Kobayashi M, Takase M, Kawase T, et al. Electrophysiological investigation of hemifacial spasm after microvascular decompression: F waves of the facial muscles, blink reflexes, and abnormal muscle responses. J Neurosurg. 86:654–661. 1997.
Article13. Isu T, Kamada K, Mabuchi S, Kitaoka A, Ito T, Koiwa M, et al. Intraoperative monitoring by facial electromyographic responses during microvascular decompressive surgery for hemifacial spasm. Acta Neurochir (Wien). 138:19–23. discussion 23. 1996.
Article14. Jo KW, Kim JW, Kong DS, Hong SH, Park K. The patterns and risk factors of hearing loss following microvascular decompression for hemifacial spasm. Acta Neurochir (Wien). 153:1023–1030. 2011.
Article15. Joo BE, Park SK, Cho KR, Kong DS, Seo DW, Park K. Real-time intraoperative monitoring of brainstem auditory evoked potentials during microvascular decompression for hemifacial spasm. J Neurosurg. 125:1061–1067. 2016.
Article16. Joo WI, Lee KJ, Park HK, Chough CK, Rha HK. Prognostic value of intraoperative lateral spread response monitoring during microvascular decompression in patients with hemifacial spasm. J Clin Neurosci. 15:1335–1339. 2008.
Article17. Kameyama S, Masuda H, Shirozu H, Ito Y, Sonoda M, Kimura J. Ephaptic transmission is the origin of the abnormal muscle response seen in hemifacial spasm. Clin Neurophysiol. 127:2240–2245. 2016.
Article18. Kim CH, Kong DS, Lee JA, Park K. The potential value of the disappearance of the lateral spread response during microvascular decompression for predicting the clinical outcome of hemifacial spams: a prospective study. Neurosurgery. 67:1581–1588. 2010.
Article19. Kim HR, Rhee DJ, Kong DS, Park K. Prognostic factors of hemifacial spasm after microvascular decompression. J Korean Neurosurg Soc. 45:336–340. 2009.
Article20. Kimura J. Current understanding of F-wave physiology in the clinical domain. Suppl Clin Neurophysiol. 59:299–303. 2006.21. Lee S, Park SK, Lee JA, Joo BE, Kong DS, Seo DW, et al. A new method for monitoring abnormal muscle response in hemifacial spasm: a prospective study. Clin Neurophysiol. 129:1490–1495. 2018.
Article22. Legatt AD. Mechanisms of intraoperative brainstem auditory evoked potential changes. J Clin Neurophysiol. 19:396–408. 2002.
Article23. Martin WH, Stecker MM. ASNM position statement: intraoperative monitoring of auditory evoked potentials. J Clin Monit Comput. 22:75–85. 2008.
Article24. Møller AR. Vascular compression of cranial nerves: II: pathophysiology. Neurol Res. 21:439–443. 1999.
Article25. Møller AR, Jannetta PJ. Microvascular decompression in hemifacial spasm: intraoperative electrophysiological observations. Neurosurgery. 16:612–618. 1985.
Article26. Møller AR, Jannetta PJ. Monitoring facial EMG responses during microvascular decompression operations for hemifacial spasm. J Neurosurg. 66:681–685. 1987.
Article27. Mooij JJ, Mustafa MK, van Weerden TW. Hemifacial spasm: intraoperative electromyographic monitoring as a guide for microvascular decompression. Neurosurgery. 49:1365–1370. discussion 1370-1371. 2001.
Article28. Nielsen VK. Pathophysiology of hemifacial spasm: I. Ephaptic transmission and ectopic excitation. Neurology. 34:418–426. 1984.
Article29. Oge AE, Yayla V, Demir GA, Eraksoy M. Excitability of facial nucleus and related brain-stem reflexes in hemifacial spasm, post-facial palsy synkinesis and facial myokymia. Clin Neurophysiol. 116:1542–1554. 2005.
Article30. Park SK, Joo BE, Lee S, Lee JA, Hwang JH, Kong DS, et al. The critical warning sign of real-time brainstem auditory evoked potentials during microvascular decompression for hemifacial spasm. Clin Neurophysiol. 129:1097–1102. 2018.
Article31. Polo G, Fischer C, Sindou MP, Marneffe V. Brain auditory evoked potentialmonitoring during microvascular decompression for hemifacial spasm: intra-operative brainstem auditory evoked potential changes and warning values to prevent hearing loss—prospective study in a consecutive series of 84 patients. Neurosurgery. 54:97–106. 2004.
Article32. Prell J, Rampp S, Rachinger J, Scheller C, Naraghi R, Strauss C. Spontaneous electromyographic activity during microvascular decompression in trigeminal neuralgia. J Clin Neurophysiol. 25:225–232. 2008.
Article33. Romstöck J, Strauss C, Fahlbusch R. Continuous electromyography monitoring of motor cranial nerves during cerebellopontine angle surgery. J Neurosurg. 93:586–593. 2000.
Article34. Samii M, Günther T, Iaconetta G, Muehling M, Vorkapic P, Samii A. Microvascular decompression to treat hemifacial spasm: long-term results for a consecutive series of 143 patients. Neurosurgery. 50:712–718. discussion 718-719. 2002.
Article35. Shin JC, Chung UH, Kim YC, Park CI. Prospective study of microvascular decompression in hemifacial spasm. Neurosurgery. 40:730–734. discussion 734-735. 1997.
Article36. Sindou M, Mercier P. Microvascular decompression for hemifacial spasm : surgical techniques and intraoperative monitoring. Neurochirurgie. 64:133–143. 2018.
Article37. Sindou MP. Microvascular decompression for primary hemifacial spasm. Impor-tance of intraoperative neurophysiological monitoring. Acta Neurochir Wien. 147:1019–1026. discussion 1026. 2005.
Article38. Son BC, Ko HC, Choi JG. Intraoperative monitoring of Z-L response (ZLR) and abnormal muscle response (AMR) during microvascular decompression for hemifacial spasm. Interpreting the role of ZLR. Acta Neurochir (Wien). 160:963–970. 2018.
Article39. Thirumala PD, Carnovale G, Habeych ME, Crammond DJ, Balzer JR. Diagnostic accuracy of brainstem auditory evoked potentials during microvascular decompression. Neurology. 83:1747–1752. 2014.
Article40. Thirumala PD, Wang X, Shah A, Habeych M, Crammond D, Balzer JR, et al. Clinical impact of residual lateral spread response after adequate microvascular decompression for hemifacial spasm: a retrospective analysis. Br J Neurosurg. 15:818–822. 2015.
Article41. Tobishima H, Hatayama T, Ohkuma H. Relation between the persistence of an abnormal muscle response and the long-term clinical course after microvascular decompression for hemifacial spasm. Neurol Med Chir (Tokyo). 54:474–482. 2014.
Article42. von Eckardstein K, Harper C, Castner M, Link M. The significance of intraoperative electromyographic “lateral spread” in predicting outcome of microvascular decompression for hemifacial spasm. J Neurol Surg B Skull Base. 75:198–203. 2014.
Article43. Wilkinson MF, Kaufmann AM. Monitoring of facial muscle motor evoked potentials during microvascular decompression for hemifacial spasm: evidence of changes in motor neuron excitability. J Neurosurg. 103:64–69. 2005.
Article44. Xu XL, Zhen XK, Yuan Y, Liu HJ, Liu J, Xu J, et al. Long-term outcome of repeat microvascular decompression for hemifacial spasm. World Neurosurg. 110:e989–e997. 2018.
Article45. Yamashita S, Kawaguchi T, Fukuda M, Suzuki K, Watanabe M, Tanaka R, et al. Lateral spread response elicited by double stimulation in patients with hemifacial spasm. Muscle Nerve. 25:845–849. 2002.
Article46. Yang M, Zheng X, Ying T, Zhu J, Zhang W, Yang X, et al. Combined intraoperative monitoring of abnormal muscle response and Z-L response for hemifacial spasm with tandem compression type. Acta Neurochir (Wien). 156:1161–1166. discussion 1166. 2014.
Article47. Zheng X, Hong W, Tang Y, Ying T, Wu Z, Shang M, et al. Discovery of a new waveform for intraoperative monitoring of hemifacial spasms. Acta Neurochir (Wien). 154:799–805. 2012.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Intraoperative monitoring of microvascular decompression in hemifacial spasm
- Efficacy of Intraoperative Facial Electromyographic Monitoring in Patients with Hemifacial Spasm
- Intraoperative Facial EMG Monitoring during Decompression Operation for Hemifacial Spasm
- Microvascular Decompression for Tinnitus
- Microvascular Decompression for Hemifacial Spasm