Hospital-Based Korean Diabetes Prevention Study: A Prospective, Multi-Center, Randomized, Open-Label Controlled Study
- Affiliations
-
- 1Department of Endocrinology and Metabolism, Kyung Hee University School of Medicine, Seoul, Korea. jtwoomd@khmc.or.kr
- KMID: 2460972
- DOI: http://doi.org/10.4093/dmj.2018.0033
Abstract
- BACKGROUND
The prevalence of diabetes mellitus (DM) continues to increase, and the disease burden is the highest of any medical condition in Korea. However, large-scale clinical studies have not yet conducted to establish the basis for diabetes prevention in Korea.
METHODS
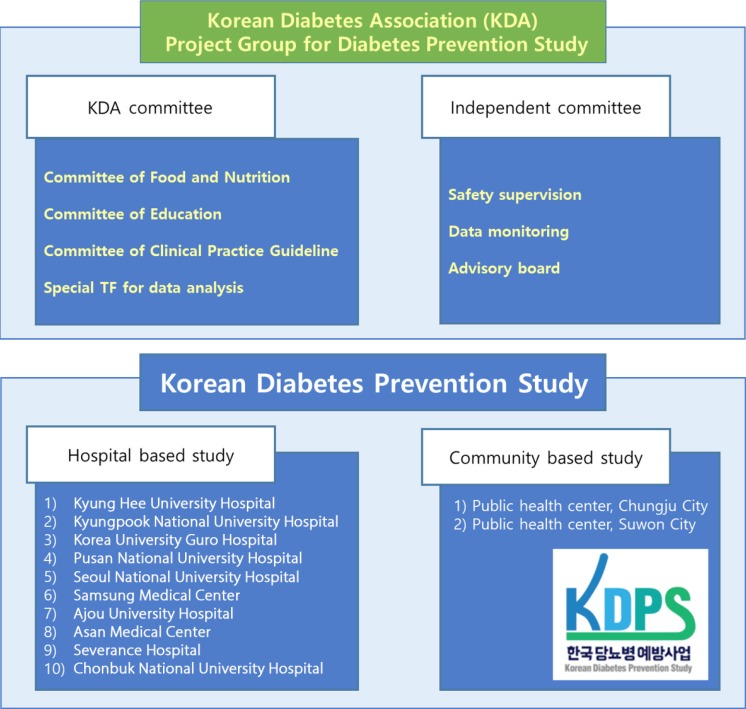
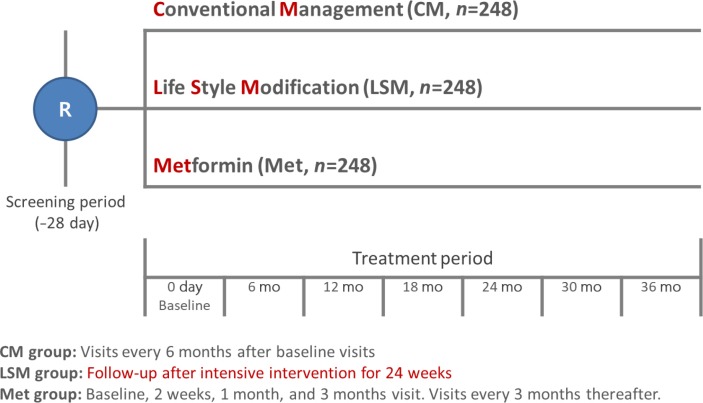
The hospital-based Korean Diabetes Prevention Study (H-KDPS) is a prospective, multi-center, randomized, open-label controlled study conducted at university hospitals for the purpose of gathering data to help in efforts to prevent type 2 DM. Ten university hospitals are participating, and 744 subjects will be recruited. The subjects are randomly assigned to the standard care group, lifestyle modification group, or metformin group, and their clinical course will be observed for 36 months.
RESULTS
All intervention methodologies were developed, validated, and approved by Korean Diabetes Association (KDA) multi-disciplinary team members. The standard control group will engage in individual education based on the current KDA guidelines, and the lifestyle modification group will participate in a professionally guided healthcare intervention aiming for ≥5% weight loss. The metformin group will begin dosing at 250 mg/day, increasing to a maximum of 1,000 mg/day. The primary endpoint of this study is the cumulative incidence of DM during the 3 years after randomization.
CONCLUSION
The H-KDPS study is the first large-scale clinical study to establish evidence-based interventions for the prevention of type 2 DM in Koreans. The evidence gathered by this study will be useful for enhancing the health of Koreans and improving the stability of the Korean healthcare system (Trial registration: CRIS KCT0002260, NCT02981121).
Keyword
MeSH Terms
Figure
Cited by 5 articles
-
Development and Validation of a Deep Learning Based Diabetes Prediction System Using a Nationwide Population-Based Cohort
Sang Youl Rhee, Ji Min Sung, Sunhee Kim, In-Jeong Cho, Sang-Eun Lee, Hyuk-Jae Chang
Diabetes Metab J. 2021;45(4):515-525. doi: 10.4093/dmj.2020.0081.2021 Clinical Practice Guidelines for Diabetes Mellitus in Korea
Kyu Yeon Hur, Min Kyong Moon, Jong Suk Park, Soo-Kyung Kim, Seung-Hwan Lee, Jae-Seung Yun, Jong Ha Baek, Junghyun Noh, Byung-Wan Lee, Tae Jung Oh, Suk Chon, Ye Seul Yang, Jang Won Son, Jong Han Choi, Kee Ho Song, Nam Hoon Kim, Sang Yong Kim, Jin Wha Kim, Sang Youl Rhee, You-Bin Lee, Sang-Man Jin, Jae Hyeon Kim, Chong Hwa Kim, Dae Jung Kim, SungWan Chun, Eun-Jung Rhee, Hyun Min Kim, Hyun Jung Kim, Donghyun Jee, Jae Hyun Kim, Won Seok Choi, Eun-Young Lee, Kun-Ho Yoon, Seung-Hyun Ko
Diabetes Metab J. 2021;45(4):461-481. doi: 10.4093/dmj.2021.0156.Short-Term Effects of the Internet-Based Korea Diabetes Prevention Study: 6-Month Results of a Community-Based Randomized Controlled Trial
Jin-Hee Lee, Sun-Young Lim, Seon-Ah Cha, Chan-Jung Han, Ah Reum Jung, Kook-Rye Kim, Kun-Ho Yoon, Seung-Hyun Ko
Diabetes Metab J. 2021;45(6):960-965. doi: 10.4093/dmj.2020.0225.2021 Clinical Practice Guidelines for Diabetes Mellitus in Korea
Seung-Hyun Ko
J Korean Diabetes. 2021;22(4):244-249. doi: 10.4093/jkd.2021.22.4.244.2023 Clinical Practice Guidelines for Diabetes Management in Korea: Full Version Recommendation of the Korean Diabetes Association
Jun Sung Moon, Shinae Kang, Jong Han Choi, Kyung Ae Lee, Joon Ho Moon, Suk Chon, Dae Jung Kim, Hyun Jin Kim, Ji A Seo, Mee Kyoung Kim, Jeong Hyun Lim, Yoon Ju Song, Ye Seul Yang, Jae Hyeon Kim, You-Bin Lee, Junghyun Noh, Kyu Yeon Hur, Jong Suk Park, Sang Youl Rhee, Hae Jin Kim, Hyun Min Kim, Jung Hae Ko, Nam Hoon Kim, Chong Hwa Kim, Jeeyun Ahn, Tae Jung Oh, Soo-Kyung Kim, Jaehyun Kim, Eugene Han, Sang-Man Jin, Jaehyun Bae, Eonju Jeon, Ji Min Kim, Seon Mee Kang, Jung Hwan Park, Jae-Seung Yun, Bong-Soo Cha, Min Kyong Moon, Byung-Wan Lee
Diabetes Metab J. 2024;48(4):546-708. doi: 10.4093/dmj.2024.0249.
Reference
-
1. Kim DJ. The epidemiology of diabetes in Korea. Diabetes Metab J. 2011; 35:303–308. PMID: 21977448.
Article2. Noh J. The diabetes epidemic in Korea. Endocrinol Metab (Seoul). 2016; 31:349–353. PMID: 27586447.
Article3. Bommer C, Heesemann E, Sagalova V, Manne-Goehler J, Atun R, Barnighausen T, Vollmer S. The global economic burden of diabetes in adults aged 20-79 years: a cost-of-illness study. Lancet Diabetes Endocrinol. 2017; 5:423–430. PMID: 28456416.
Article4. Lee KW. Costs of diabetes mellitus in Korea. Diabetes Metab J. 2011; 35:567–570. PMID: 22247897.
Article5. Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, Hu ZX, Lin J, Xiao JZ, Cao HB, Liu PA, Jiang XG, Jiang YY, Wang JP, Zheng H, Zhang H, Bennett PH, Howard BV. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997; 20:537–544. PMID: 9096977.
Article6. Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M. Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001; 344:1343–1350. PMID: 11333990.
Article7. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002; 346:393–403. PMID: 11832527.
Article8. Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M. STOP-NIDDM Trail Research Group. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet. 2002; 359:2072–2077. PMID: 12086760.
Article9. DREAM (Diabetes REduction Assessment with ramipril and rosiglitazone Medication) Trial Investigators. Gerstein HC, Yusuf S, Bosch J, Pogue J, Sheridan P, Dinccag N, Hanefeld M, Hoogwerf B, Laakso M, Mohan V, Shaw J, Zinman B, Holman RR. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet. 2006; 368:1096–1105. PMID: 16997664.10. Kawamori R, Tajima N, Iwamoto Y, Kashiwagi A, Shimamoto K, Kaku K. Voglibose Ph-3 Study Group. Voglibose for prevention of type 2 diabetes mellitus: a randomised, double-blind trial in Japanese individuals with impaired glucose tolerance. Lancet. 2009; 373:1607–1614. PMID: 19395079.
Article11. DeFronzo RA, Tripathy D, Schwenke DC, Banerji M, Bray GA, Buchanan TA, Clement SC, Henry RR, Hodis HN, Kitabchi AE, Mack WJ, Mudaliar S, Ratner RE, Williams K, Stentz FB, Musi N, Reaven PD. ACT NOW Study. Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med. 2011; 364:1104–1115. PMID: 21428766.
Article12. Li G, Zhang P, Wang J, Gregg EW, Yang W, Gong Q, Li H, Li H, Jiang Y, An Y, Shuai Y, Zhang B, Zhang J, Thompson TJ, Gerzoff RB, Roglic G, Hu Y, Bennett PH. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet. 2008; 371:1783–1789. PMID: 18502303.
Article13. Gong Q, Gregg EW, Wang J, An Y, Zhang P, Yang W, Li H, Li H, Jiang Y, Shuai Y, Zhang B, Zhang J, Gerzoff RB, Roglic G, Hu Y, Li G, Bennett PH. Long-term effects of a randomised trial of a 6-year lifestyle intervention in impaired glucose tolerance on diabetes-related microvascular complications: the China Da Qing Diabetes Prevention Outcome Study. Diabetologia. 2011; 54:300–307. PMID: 21046360.
Article14. Diabetes Prevention Program Research Group. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol. 2015; 3:866–875. PMID: 26377054.15. Diabetes Prevention Program Research Group. Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, Brenneman AT, Brown-Friday JO, Goldberg R, Venditti E, Nathan DM. 10-Year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009; 374:1677–1686. PMID: 19878986.16. Li G, Zhang P, Wang J, An Y, Gong Q, Gregg EW, Yang W, Zhang B, Shuai Y, Hong J, Engelgau MM, Li H, Roglic G, Hu Y, Bennett PH. Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: a 23-year follow-up study. Lancet Diabetes Endocrinol. 2014; 2:474–480. PMID: 24731674.
Article17. Baker MK, Simpson K, Lloyd B, Bauman AE, Singh MA. Behavioral strategies in diabetes prevention programs: a systematic review of randomized controlled trials. Diabetes Res Clin Pract. 2011; 91:1–12. PMID: 20655610.
Article18. Ackermann RT, Marrero DG. Adapting the Diabetes Prevention Program lifestyle intervention for delivery in the community: the YMCA model. Diabetes Educ. 74; 33:6974–75. 77–78. PMID: 17272794.19. Chan AW, Tetzlaff JM, Gotzsche PC, Altman DG, Mann H, Berlin JA, Dickersin K, Hrobjartsson A, Schulz KF, Parulekar WR, Krleza-Jeric K, Laupacis A, Moher D. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013; 346:e7586. PMID: 23303884.
Article20. Cha SA, Lim SY, Kim KR, Lee EY, Kang B, Choi YH, Yoon KH, Ahn YB, Lee JH, Ko SH. Community-based randomized controlled trial of diabetes prevention study for high-risk individuals of type 2 diabetes: lifestyle intervention using web-based system. BMC Public Health. 2017; 17:387. PMID: 28476101.
Article21. Korean Diabetes Association. 2015 Treatment Guideline for Diabetes. updated 2016 Oct 12. Available from: http://www.diabetes.or.kr/pro/publish/guide.php?code=guide&number=638&mode=view.22. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004; 27:1487–1495. PMID: 15161807.
Article23. Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V. Indian Diabetes Prevention Programme (IDPP). The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia. 2006; 49:289–297. PMID: 16391903.
Article24. Um HD, Lee DC, Lee SY, Kim YS. A prospective cohort study of exercise and the incidence of type 2 diabetes in impaired fasting glucose group. J Prev Med Public Health. 2008; 41:45–50. PMID: 18250605.
Article25. Han SJ, Kim HJ, Kim DJ, Lee KW, Cho NH. Incidence and predictors of type 2 diabetes among Koreans: a 12-year follow up of the Korean Genome and Epidemiology Study. Diabetes Res Clin Pract. 2017; 123:173–180. PMID: 28043048.
Article26. Kweon S, Kim Y, Jang MJ, Kim Y, Kim K, Choi S, Chun C, Khang YH, Oh K. Data resource profile: the Korea National Health and Nutrition Examination Survey (KNHANES). Int J Epidemiol. 2014; 43:69–77. PMID: 24585853.
Article27. Shin HY, Lee JY, Song J, Lee S, Lee J, Lim B, Kim H, Huh S. Cause-of-death statistics in the Republic of Korea, 2014. J Korean Med Assoc. 2016; 59:221–232.
Article28. Yoon J, Oh IH, Seo H, Kim EJ, Gong YH, Ock M, Lim D, Lee WK, Lee YR, Kim D, Jo MW, Park H, Yoon SJ. Disability-adjusted life years for 313 diseases and injuries: the 2012 Korean Burden of Disease Study. J Korean Med Sci. 2016; 31(Suppl 2):S146–S157. PMID: 27775252.
Article29. Rhee SY, Woo JT. The prediabetic period: review of clinical aspects. Diabetes Metab J. 2011; 35:107–116. PMID: 21738892.
Article30. Tabak AG, Herder C, Rathmann W, Brunner EJ, Kivimaki M. Prediabetes: a high-risk state for diabetes development. Lancet. 2012; 379:2279–2290. PMID: 22683128.31. Rhee SY, Kim JY, Chon S, Hwang YC, Jeong IK, Oh S, Ahn KJ, Chung HY, Woo JT, Kim SW, Kim JW, Kim YS. The changes in early phase insulin secretion in newly diagnosed, drug naive korean prediabetes subjects. Korean Diabetes J. 2010; 34:157–165. PMID: 20617076.
Article32. Rhee SY, Woo JT, Chon S, Hwang YC, Oh S, Ahn KJ, Chung HY, Kim SW, Kim JW, Kim YS. Characteristics of insulin resistance and insulin secretory capacity in Korean subjects with IFG and IGT. Diabetes Res Clin Pract. 2010; 89:250–255. PMID: 20605249.
Article33. Forouhi NG, Luan J, Hennings S, Wareham NJ. Incidence of type 2 diabetes in England and its association with baseline impaired fasting glucose: the Ely study 1990–2000. Diabet Med. 2007; 24:200–207. PMID: 17257284.
Article34. Icks A, Rathmann W, Haastert B, Gandjour A, Holle R, John J, Giani G. KORA Study Group. Clinical and cost-effectiveness of primary prevention of type 2 diabetes in a ‘real world’ routine healthcare setting: model based on the KORA Survey 2000. Diabet Med. 2007; 24:473–480. PMID: 17381502.
Article35. Diabetes Prevention Program Research Group. The 10-year cost-effectiveness of lifestyle intervention or metformin for diabetes prevention: an intent-to-treat analysis of the DPP/DPPOS. Diabetes Care. 2012; 35:723–730. PMID: 22442395.36. Ackermann RT, Liss DT, Finch EA, Schmidt KK, Hays LM, Marrero DG, Saha C. A randomized comparative effectiveness trial for preventing type 2 diabetes. Am J Public Health. 2015; 105:2328–2334. PMID: 26378828.
Article37. Mensa-Wilmot Y, Bowen SA, Rutledge S, Morgan JM, Bonner T, Farris K, Blacher R, Rutledge G. Early results of states' efforts to support, scale, and sustain the National Diabetes Prevention Program. Prev Chronic Dis. 2017; 14:E130. PMID: 29215975.
Article38. Selvin E, Ali MK. Declines in the incidence of diabetes in the U.S.: real progress or artifact? Diabetes Care. 2017; 40:1139–1143. PMID: 28830954.39. Halliday M. First drug approved for diabetes prevention. updated 2017 May 22. Available from: https://www.mims.co.uk/first-drug-approved-diabetes-prevention/diabetes/article/1434274.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Letter: Efficacy and Safety of Biphasic Insulin Aspart 30/70 in Type 2 Diabetes Suboptimally Controlled on Oral Antidiabetic Therapy in Korea: A Multicenter, Open-Label, Single-Arm Study (Diabetes Metab J 2013;37:117-24)
- Comparison of Acarbose and Voglibose in Diabetes Patients Who Are Inadequately Controlled with Basal Insulin Treatment: Randomized, Parallel, Open-Label, Active-Controlled Study
- New Insulin Pumps and Open Source Artificial Pancreas System in Korea
- Author's Response: A Prospective, Randomized, Controlled Comparative Study of Three Energy Devices in Open Thyroid Surgery: Thunderbeat, Harmonic, and Ligasure
- Study Design and Protocol for a Randomized Controlled Trial of Enavogliflozin to Evaluate Cardiorenal Outcomes in Type 2 Diabetes (ENVELOP)