J Korean Diabetes.
2020 Dec;21(4):197-203. 10.4093/jkd.2020.21.4.197.
New Insulin Pumps and Open Source Artificial Pancreas System in Korea
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2511886
- DOI: http://doi.org/10.4093/jkd.2020.21.4.197
Abstract
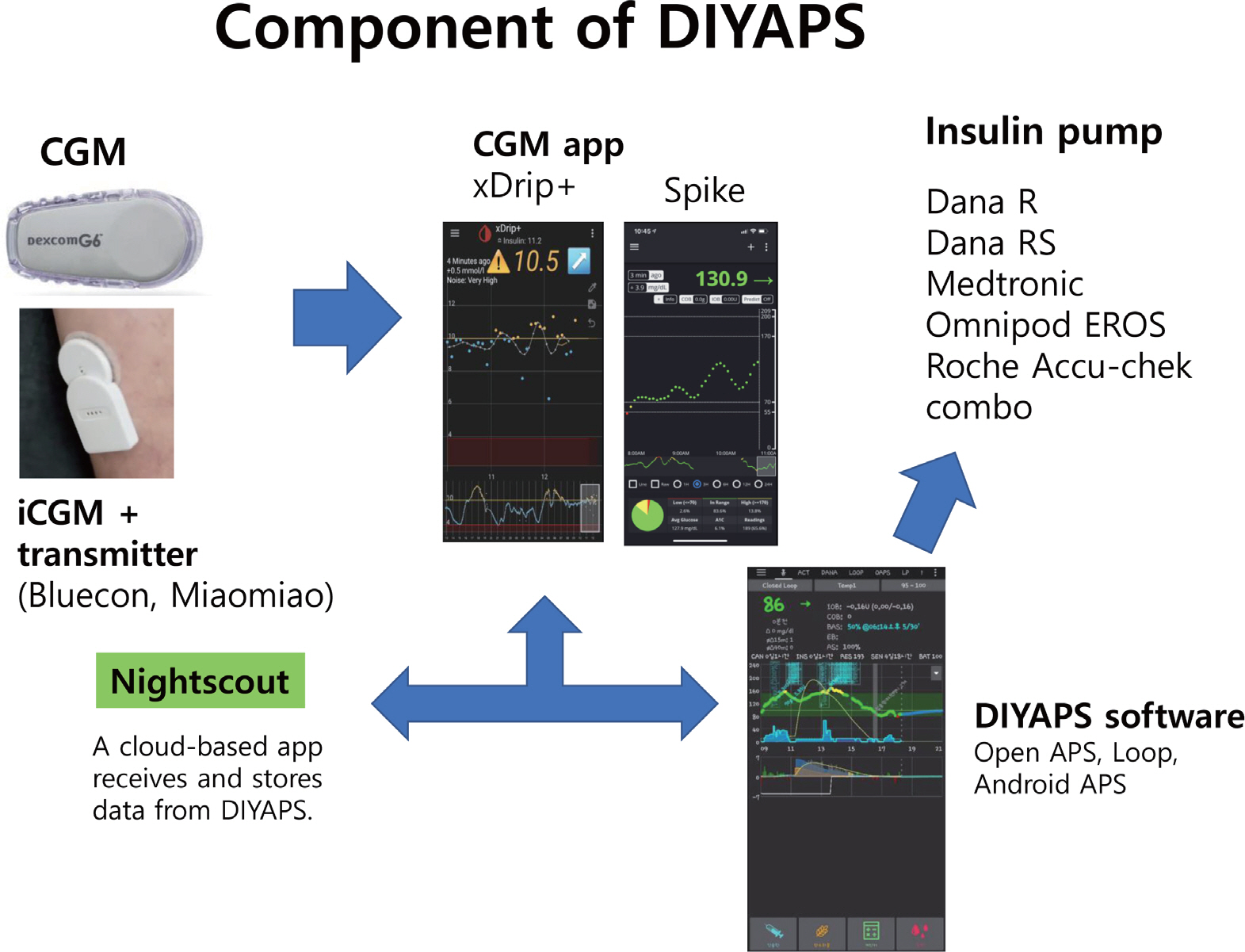
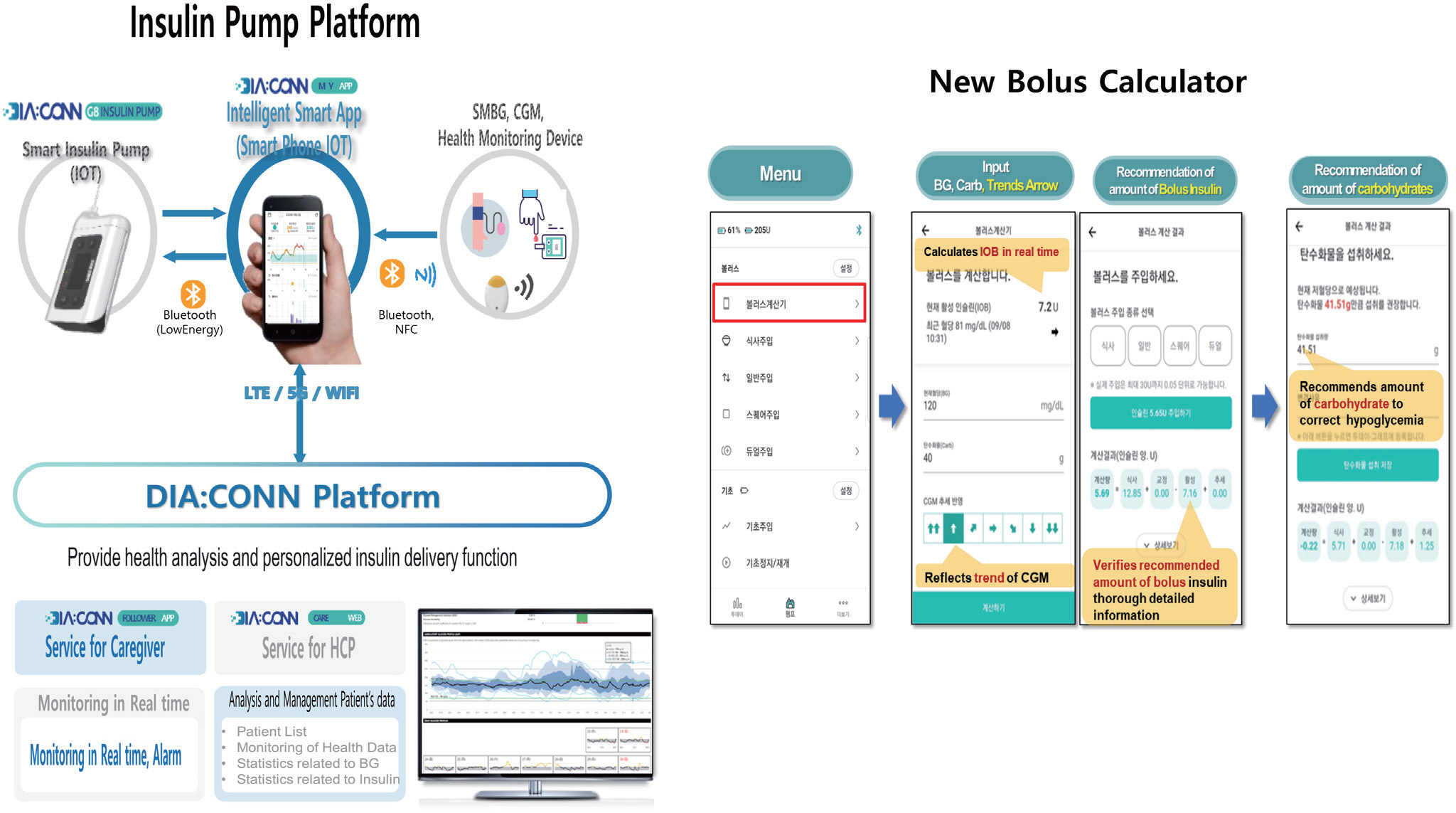
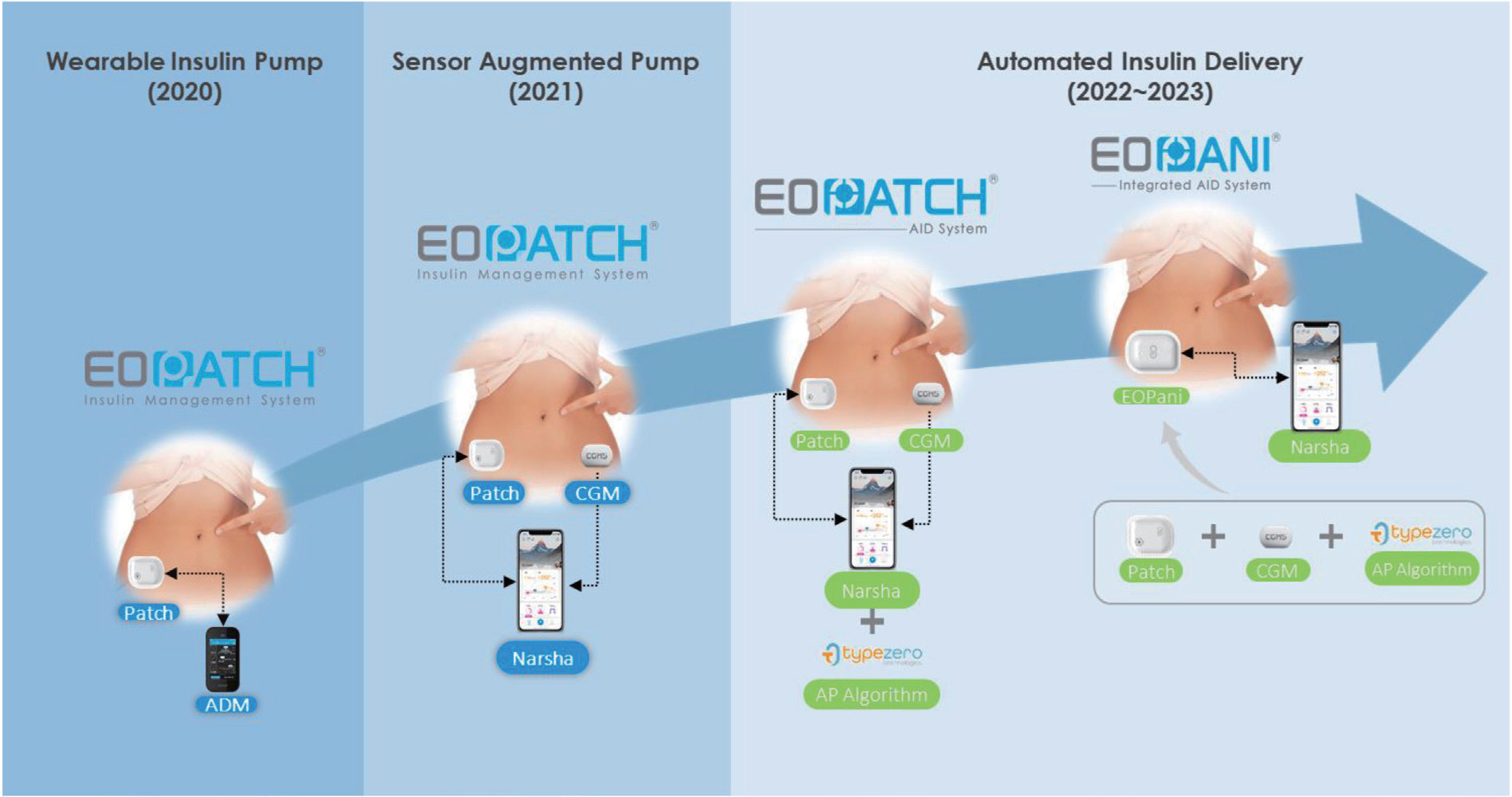
- Diabetes technology that includes artificial pancreas system (APS) has achieved remarkable progress in the past decades. The first APS, the Medtronic MiniMed 670G system introduced in 2017, and other subsequent commercial APSs have come as answers to patient and clinician prayers. However, these devices are not accessible by most persons with type 1 diabetes (T1D) in Korea. Therefore, highly motivated, and tech-savvy persons in the diabetes community have started developing open source APS (Open APS) that integrate continuous glucose monitoring (CGM), insulin pumps, and smartphone technology to run their own do-it-yourself APS (DIYAPS). Observational studies have revealed significant improvements in HbA1c levels, time in range, and quality of life after the initiation of Open APS use. However, the use of regularized CGM and an insulin pump with an unauthorized DIYAPS makes these devices off-label and this has been a matter of grave clinical concern. In addition, lack of randomized controlled trials, insurance coverage, and legal issues are barriers to the general acceptance of DIYAPS among persons with T1D. In this article, I will summarize existing DIYAPS studies including those in Korea and their concerns. In addition, I will introduce new insulin pumps available in Korea.
Keyword
Figure
Reference
-
1. Iqbal A., Novodvorsky P., Heller SR. Recent updates on type 1 diabetes mellitus management for clinicians. Diabetes Metab J. 2018. 42:3–18.
Article2. Lee YB., Han K., Kim B., Jin SM., Lee SE., Jun JE, et al. High proportion of adult cases and prevalence of metabolic syndrome in type 1 diabetes mellitus population in Korea: a nationwide study. Diabetes Metab J. 2019. 43:76–89.
Article3. Lee YB., Han K., Kim B., Lee SE., Jun JE., Ahn J, et al. Risk of early mortality and cardiovascular disease in type 1 diabetes: a comparison with type 2 diabetes, a nationwide study. Cardiovasc Diabetol. 2019. 18:157.
Article4. Lee YB., Han K., Kim B., Jun JE., Lee SE., Ahn J, et al. Risk of end-stage renal disease from chronic kidney disease defined by decreased glomerular filtration rate in type 1 diabetes: a comparison with type 2 diabetes and the effect of metabolic syndrome. Diabetes Metab Res Rev. 2019. 35:e3197.
Article5. Lewis DM. Do-it-yourself artificial pancreas system and the OpenAPS movement. Endocrinol Metab Clin North Am. 2020. 49:203–13.
Article6. Kesavadev J., Srinivasan S., Saboo B., Krishna BM., Krishnan G. The do-it-yourself artificial pancreas: a comprehensive review. Diabetes Ther. 2020. 11:1217–35.
Article7. Choi SB., Hong ES., Noh YH. Open artificial pancreas system reduced hypoglycemia and improved glycemic control in patients with type 1 diabetes. Diabetes. 2018. 67(Suppl 1):https://doi.org/10.2337/db18-964-P.
Article8. U.S. Food and Drug Administration (FDA). FDA warns against the use of unauthorized devices for diabetes management. Silver Spring, MD: FDA;2019.9. de Bock M. The ‘do it yourself’ type 1 diabetes dilemma for medical practitioners. Intern Med J. 2019. 49:559–61.
Article10. Wilmot EG., Danne T. DIY artificial pancreas systems: the clinician perspective. Lancet Diabetes Endocrinol. 2020. 8:183–5.
Article11. EOFLOW. Press release 2020 Oct 6. Available from:. https://file.irgo.co.kr/data/BOARD/ATTACH_PDF/e69fdd56-6698-4189-9340-dd1e4aa195ef.pdf. (updated 2020 Oct 6).
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Artificial Pancreas: A Concise Review
- New Technology for Type 1 Diabetes
- Current Advances of Artificial Pancreas Systems: A Comprehensive Review of the Clinical Evidence
- Do-It-Yourself Open Artificial Pancreas System in Children and Adolescents with Type 1 Diabetes Mellitus: Real-World Data
- Application of Automated Insulin Delivery in Korean Clinical Practice