Yonsei Med J.
2019 Nov;60(11):1013-1020. 10.3349/ymj.2019.60.11.1013.
A Two-DNA Methylation Signature to Improve Prognosis Prediction of Clear Cell Renal Cell Carcinoma
- Affiliations
-
- 1Diabetes Center, Zhejiang Provincial Key Laboratory of Pathophysiology, Institute of Biochemistry and Molecular Biology, Medical School of Ningbo University, Ningbo, Zhejiang, China. xiyang@nbu.edu.cn
- 2Department of Preventative Medicine, Medical School of Ningbo University, Ningbo, Zhejiang, China.
- KMID: 2460217
- DOI: http://doi.org/10.3349/ymj.2019.60.11.1013
Abstract
- PURPOSE
Effective biomarkers and models are needed to improve the prognostic prospects of clear cell renal cell carcinoma (ccRCC). The purpose of this work was to identify DNA methylation biomarkers and to evaluate the utility of DNA methylation analysis for ccRCC prognosis.
MATERIALS AND METHODS
An overview of genome-wide methylation of ccRCC tissues derived from The Cancer Genome Atlas (TCGA) database was download for analysis. DNA methylation signatures were identified using Cox regression methods. The potential clinical significance of methylation biomarkers acting as a novel prognostic markers was analyzed using the Kaplan-Meier method and receiver operating characteristic (ROC) curves.
RESULTS
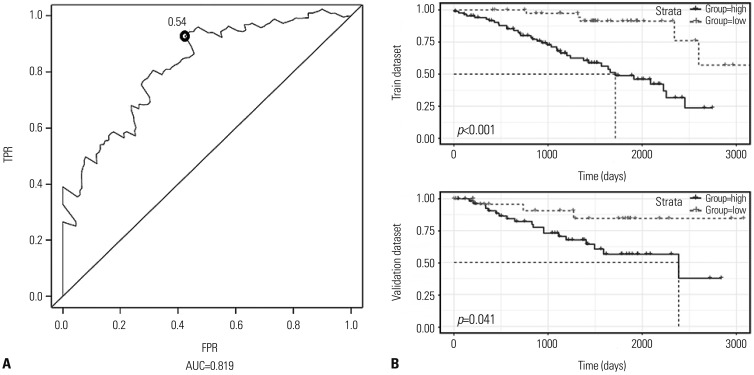
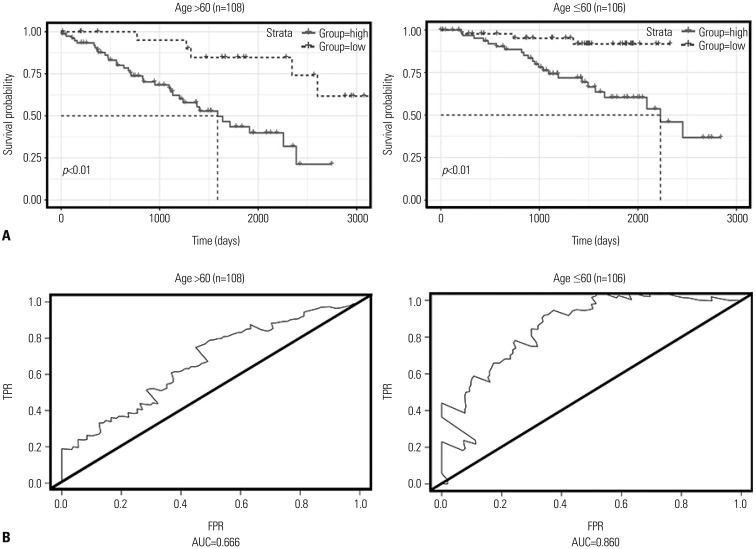
This study analyzed data for 215 patients with information on 23171 DNA methylation sites and identified a two-DNA methylation signature (cg18034859, cg24199834) with the help of a step-wise multivariable Cox regression model. The area under the curve of ROCs for the two-DNA methylation signature was 0.819. The study samples were stratified into low- and high-risk classifications based on an optimal threshold, and the two groups showed markedly different survival rates. Moreover, the two-DNA methylation marker was suitable for patients of varying ages, sex, stages (I and IV), and histologic grade (G2).
CONCLUSION
The two-DNA methylation signature was deemed to be a potential novel prognostic biomarker of use in increasing the accuracy of predicting overall survival of ccRCC patients.
Keyword
MeSH Terms
Figure
Reference
-
1. Thiesen HJ, Steinbeck F, Maruschke M, Koczan D, Ziems B, Hakenberg OW. Stratification of clear cell renal cell carcinoma (ccRCC) genomes by gene-directed copy number alteration (CNA) analysis. PLoS One. 2017; 12:e0176659. PMID: 28486536.
Article2. Ljungberg B, Bensalah K, Canfield S, Dabestani S, Hofmann F, Hora M, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol. 2015; 67:913–924. PMID: 25616710.
Article3. Young JR, Coy H, Douek M, Lo P, Sayre J, Pantuck AJ, et al. Clear cell renal cell carcinoma: identifying the gain of chromosome 12 on multiphasic MDCT. Abdom Radiol (NY). 2017; 42:236–241. PMID: 27519835.
Article4. Mehdi A, Riazalhosseini Y. Epigenome aberrations: emerging driving factors of the clear cell renal cell carcinoma. Int J Mol Sci. 2017; 18:E1774. PMID: 28812986.
Article5. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017; 67:7–30. PMID: 28055103.
Article6. Drucker BJ. Renal cell carcinoma: current status and future prospects. Cancer Treat Rev. 2005; 31:536–545. PMID: 16236454.
Article7. Scélo G, Brennan P. The epidemiology of bladder and kidney cancer. Nat Clin Pract Urol. 2007; 4:205–217. PMID: 17415353.
Article8. Pan Y, Liu G, Zhou F, Su B, Li Y. DNA methylation profiles in cancer diagnosis and therapeutics. Clin Exp Med. 2018; 18:1–14. PMID: 28752221.
Article9. van der Kooi EL, de Greef JC, Wohlgemuth M, Frants RR, van Asseldonk RJ, Blom HJ, et al. No effect of folic acid and methionine supplementation on D4Z4 methylation in patients with facioscapulohumeral muscular dystrophy. Neuromuscul Disord. 2006; 16:766–769. PMID: 17005397.
Article10. Hao X, Luo H, Krawczyk M, Wei W, Wang W, Wang J, et al. DNA methylation markers for diagnosis and prognosis of common cancers. Proc Natl Acad Sci U S A. 2017; 114:7414–7419. PMID: 28652331.
Article11. Dubrowinskaja N, Gebauer K, Peters I, Hennenlotter J, Abbas M, Scherer R, et al. Neurofilament Heavy polypeptide CpG island methylation associates with prognosis of renal cell carcinoma and prediction of antivascular endothelial growth factor therapy response. Cancer Med. 2014; 3:300–309. PMID: 24464810.12. Wang L, Gao W, Hu F, Xu Z, Wang F. MicroRNA-874 inhibits cell proliferation and induces apoptosis in human breast cancer by targeting CDK9. FEBS Lett. 2014; 588:4527–4535. PMID: 25281924.
Article13. Zhang L, Yan DL, Yang F, Wang DD, Chen X, Wu JZ, et al. DNA methylation mediated silencing of microRNA-874 is a promising diagnosis and prognostic marker in breast cancer. Oncotarget. 2017; 8:45496–45505. PMID: 28525377.
Article14. Li W, Zhang X, Lu X, You L, Song Y, Luo Z, et al. 5-Hydroxymethylcytosine signatures in circulating cell-free DNA as diagnostic biomarkers for human cancers. Cell Res. 2017; 27:1243–1257. PMID: 28925386.
Article15. Jeschke J, Bizet M, Desmedt C, Calonne E, Dedeurwaerder S, Garaud S, et al. DNA methylation-based immune response signature improves patient diagnosis in multiple cancers. J Clin Invest. 2017; 127:3090–3102. PMID: 28714863.
Article16. Zhang W, Yan W, You G, Bao Z, Wang Y, Liu Y, et al. Genome-wide DNA methylation profiling identifies ALDH1A3 promoter methylation as a prognostic predictor in G-CIMP-primary glioblastoma. Cancer Lett. 2013; 328:120–125. PMID: 22960273.17. Shukla S, Pia Patric IR, Thinagararjan S, Srinivasan S, Mondal B, Hegde AS, et al. A DNA methylation prognostic signature of glioblastoma: identification of NPTX2-PTEN-NF-κB nexus. Cancer Res. 2013; 73:6563–6573. PMID: 24078801.
Article18. Sandoval J, Mendez-Gonzalez J, Nadal E, Chen G, Carmona FJ, Sayols S, et al. A prognostic DNA methylation signature for stage I non-small-cell lung cancer. J Clin Oncol. 2013; 31:4140–4147. PMID: 24081945.
Article19. Evelönn EA, Landfors M, Haider Z, Köhn L, Ljungberg B, Roos G, et al. DNA methylation associates with survival in non-metastatic clear cell renal cell carcinoma. BMC Cancer. 2019; 19:65. PMID: 30642274.
Article20. Peters I, Dubrowinskaja N, Hennenlotter J, Antonopoulos WI, Von Klot CAJ, Tezval H, et al. DNA methylation of neural EGFL like 1 (NELL1) is associated with advanced disease and the metastatic state of renal cell cancer patients. Oncol Rep. 2018; 40:3861–3868. PMID: 30272321.21. Dagher J, Delahunt B, Rioux-Leclercq N, Egevad L, Srigley JR, Coughlin G, et al. Clear cell renal cell carcinoma: validation of World Health Organization/International Society of Urological Pathology grading. Histopathology. 2017; 71:918–925. PMID: 28718911.
Article22. Delahunt B, Cheville C, Martignoni G, Humphrey PA, Magi-Galluzzi C, McKenney J, et al. The International Society of Urological Pathology (ISUP) grading system for renal cell carcinoma and other prognostic parameters. Am J Surg Pathol. 2013; 37:1490–1504. PMID: 24025520.
Article23. Cho HJ, Yu A, Kim S, Kang J, Hong SM. Robust likelihood-based survival modeling with microarray data. J Stat Softw. 2009; 29:1–16.
Article24. Jung EJ, Lee HJ, Kwak C, Ku JH, Moon KC. Young age is independent prognostic factor for cancer-specific survival of low-stage clear cell renal cell carcinoma. Urology. 2009; 73:137–141. PMID: 18950844.
Article25. Ricketts CJ, Linehan WM. Gender specific mutation incidence and survival associations in Clear Cell Renal Cell Carcinoma (ccRCC). PLoS One. 2015; 10:e0140257. PMID: 26484545.
Article26. Ebru T, Fulya OP, Hakan A, Vuslat YC, Necdet S, Nuray C, et al. Analysis of various potential prognostic markers and survival data in clear cell renal cell carcinoma. Int Braz J Urol. 2017; 43:440–454. PMID: 27583351.
Article27. Cheville JC, Blute ML, Zincke H, Lohse CM, Weaver AL. Stage pT1 conventional (clear cell) renal cell carcinoma: pathological features associated with cancer specific survival. J Urol. 2001; 166:453–456. PMID: 11458046.
Article28. Zhan Y, Guo W, Zhang Y, Wang Q, Xu XJ, Zhu L. A five-gene signature predicts prognosis in patients with kidney renal clear cell carcinoma. Comput Math Methods Med. 2015; 2015:842784. PMID: 26539246.
Article29. Liang B, Zhao J, Wang X. A three-microRNA signature as a diagnostic and prognostic marker in clear cell renal cancer: an In Silico analysis. PLoS One. 2017; 12:e0180660. PMID: 28662155.
Article30. Song J, Peng J, Zhu C, Bai G, Liu Y, Zhu J, et al. Identification and validation of two novel prognostic lncRNAs in kidney renal clear cell carcinoma. Cell Physiol Biochem. 2018; 48:2549–2562. PMID: 30121668.
Article31. Xu D, Zhang S, Zhang S, Liu H, Li P, Yu L, et al. NOD2 maybe a biomarker for the survival of kidney cancer patients. Oncotarget. 2017; 8:101489–101499. PMID: 29254180.
Article32. Dai W, Teodoridis JM, Zeller C, Graham J, Hersey J, Flanagan JM, et al. Systematic CpG islands methylation profiling of genes in the wnt pathway in epithelial ovarian cancer identifies biomarkers of progression-free survival. Clin Cancer Res. 2011; 17:4052–4062. PMID: 21459799.
Article33. Zuo S, Wang L, Wen Y, Dai G. Identification of a universal 6-lncRNA prognostic signature for three pathologic subtypes of renal cell carcinoma. J Cell Biochem. 2018; 10. 30. [Epub]. Available at: . DOI: 10.1002/jcb.28012.
Article34. Wu F, Kaczynski TJ, Sethuramanujam S, Li R, Jain V, Slaughter M, et al. Two transcription factors, Pou4f2 and Isl1, are sufficient to specify the retinal ganglion cell fate. Proc Natl Acad Sci U S A. 2015; 112:E1559–E1568. PMID: 25775587.
Article35. Maskell LJ, Mahadeo AV, Budhram-Mahadeo VS. POU4F2/Brn-3b transcription factor is associated with survival and drug resistance in human ovarian cancer cells. Oncotarget. 2018; 9:36770–36779. PMID: 30613365.
Article36. Reinert T, Borre M, Christiansen A, Hermann GG, Ørntoft TF, Dyrskjøt L. Diagnosis of bladder cancer recurrence based on urinary levels of EOMES, HOXA9, POU4F2, TWIST1, VIM, and ZNF154 hypermethylation. PLoS One. 2012; 7:e46297. PMID: 23056278.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Screening of Methylation Gene Sites as Prognostic Signature in Lung Adenocarcinoma
- The Relationship between RUNX3 Inactivation and Its Pathological Features in Renal Cell Carcinoma
- A Case of Papillary Type of Renal Cell Carcinoma after Renal Injury in a Child
- CT-Based Radiomics Signature for Preoperative Prediction of Coagulative Necrosis in Clear Cell Renal Cell Carcinoma
- Methylation Markers in Renal Cell Carcinoma