Anat Cell Biol.
2019 Sep;52(3):324-332. 10.5115/acb.19.009.
Influence of traditional medicines on the activity of keratinocytes in wound healing: an in-vitro study
- Affiliations
-
- 1Department of Anatomy, Kasturba Medical College Manipal, Manipal Academy of Higher Education, Manipal, India.
- 2Department of Anatomy, RAK College of Medical Sciences, RAK Medical and Health Sciences University, Ras Al Khaimah, UAE. kumarbhat@rakmhsu.ac.ae
- 3Department of Pharmacology, Manipal College of Pharmaceutical Sciences, Manipal Academy of Higher Education, Manipal, India.
- KMID: 2459548
- DOI: http://doi.org/10.5115/acb.19.009
Abstract
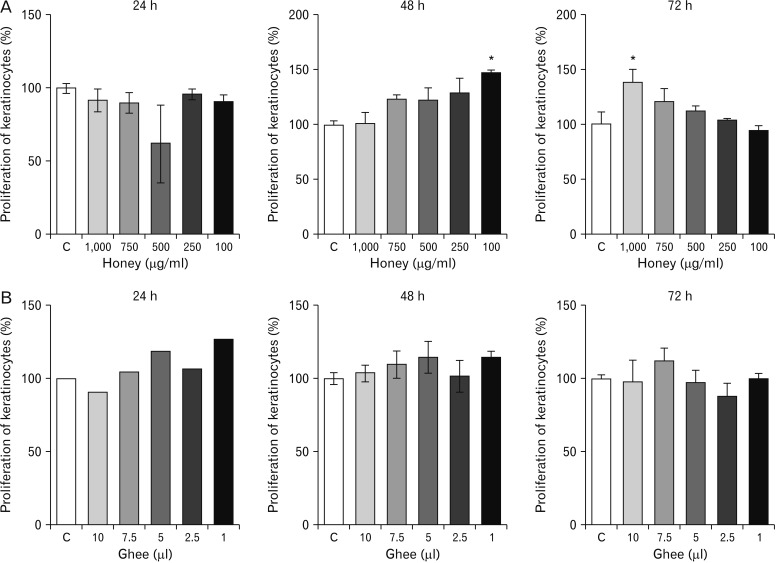
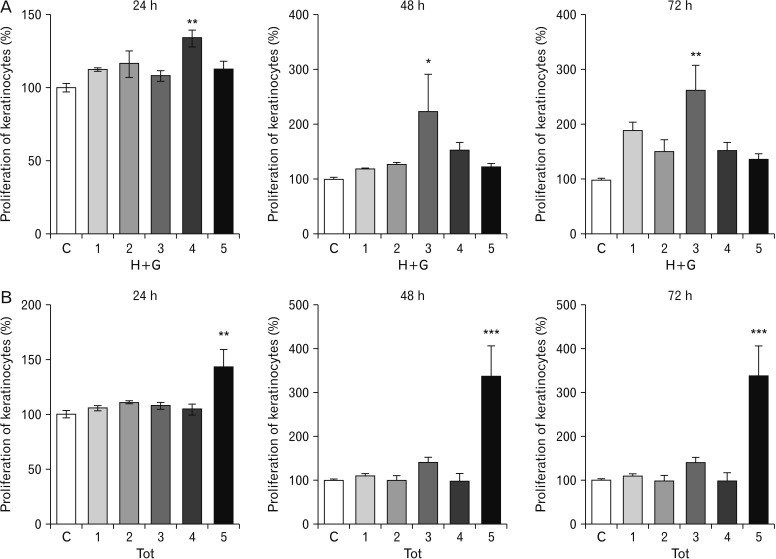
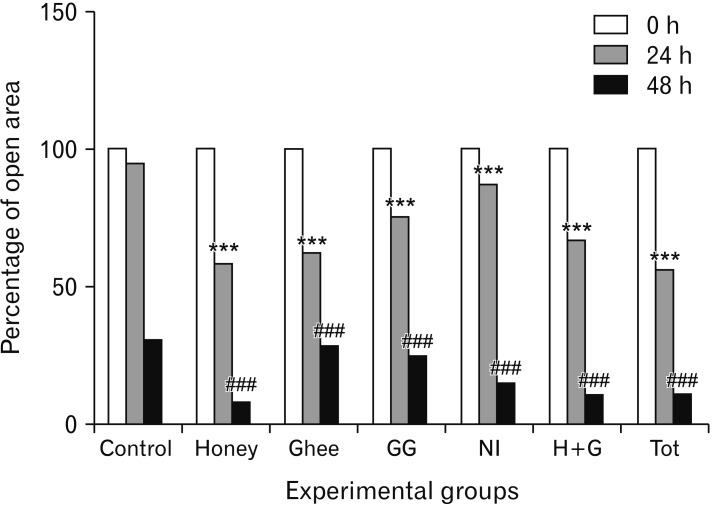
- Natural medicinal systems such as Ayurveda and folk medicine has remedies for wound management. However, the exact cellular and extracellular mechanisms involved in the healing process and its influence on keratinocytes is less discussed. Therefore, the present study was designed to evaluate the effect of certain natural wound healing medicines on the biology of the keratinocytes/HaCaT cells. Test materials such as honey (H), ghee (G), aqueous extracts of roots of Glycyrrhiza glabra (GG) and leaves of Nerium indicum (NI) were considered. The HaCaT cells were treated with the test materials singly and in combinations (H+G, all combined [Tot]) for a specific period (24, 48, and 72 hours). The cells were then subjected to cytotoxicity/proliferation and migration/scratch assays. All the test materials, except NI, were non-cytotoxic and showed increased cell proliferation at variable concentrations. Significant observations were made in the groups treated with honey (100 µg/ml at 48 hours, P<0.05; 1,000 µg/ml at 72 hours, P<0.05), GG (all concentrations at 48 hours, P<0.05; 750 µg/ml at 72 hours, P<0.05), H+G (250 µg/ml at 24 hours, P<0.001; 500 µg/ml at 48 and 72 hours, P<0.05), and Tot (50 µg/ml at 24, 48 and 72 hours, P<0.01). In the in-vitro wound healing assay, all the treated groups showed significant migration and narrowing of the scratch area by 24 and 48 hours (P<0.001) compared to control. The results obtained from the present study signifies the positive influence of these natural wound healing compounds on keratinocytes/HaCaT cells.
Keyword
MeSH Terms
Figure
Reference
-
1. Pastar I, Stojadinovic O, Tomic-Canic M. Role of keratinocytes in healing of chronic wounds. Surg Technol Int. 2008; 17:105–112. PMID: 18802889.2. Isaac C, Paggiaro AO, Aldunate JL, Herson MR, Altran SC, Mathor MB, Ferreira MC. Role of keratinocytes in wound contraction: an impact assessment using a model of collagen matrix populated with fibroblasts. Rev Bras Cir Plast. 2011; 26:402–406.3. Kotian SR, Pai KS, Nayak J, Bangera H, Prasad K, Bhat KM. Biomechanical, biochemical and histological evidences for wound healing properties of Indian traditional medicines. Int J Pharm Pharm Sci. 2015; 7:163–171.4. Prasad V, Dorle AK. Evaluation of ghee based formulation for wound healing activity. J Ethnopharmacol. 2006; 107:38–47. PMID: 16546334.5. Molan PC, Betts JA. Clinical usage of honey as a wound dressing: an update. J Wound Care. 2004; 13:353–356. PMID: 15517742.6. Molan PC. The evidence supporting the use of honey as a wound dressing. Int J Low Extrem Wounds. 2006; 5:40–54. PMID: 16543212.7. Oloumi MM, Derakhshanfar A, Nikpoor A. Healing potential of liquorice root extract on dermal wounds in rats. J Vet Res. 2007; 62:147–154.8. Sravanthi KC, Manthri S, Srilakshmi S, Ashajyothi V. Wound healing herbs: a review. Int J Pharm Technol. 2010; 2:603–624.9. Kotian SR, Padma D, Madhukar R, Bhat KM. Effect of natural medicines on dermal fibroblasts in wound healing: an in-vitro study. Adv Sci Lett. 2017; 23:1949–1956.10. Davis H. Bentley's textbook of pharmaceutics. 6th ed. London: Balliere, Tindall and Co;1956. p. 272–230.11. Schurer N, Kohne A, Schliep V, Barlag K, Goerz G. Lipid composition and synthesis of HaCaT cells, an immortalized human keratinocyte line, in comparison with normal human adult keratinocytes. Exp Dermatol. 1993; 2:179–185. PMID: 8162337.12. Ali A, Kim MJ, Kim MY, Lee HJ, Roh GS, Kim HJ, Cho GJ, Choi WS. Quercetin induces cell death in cervical cancer by reducing O-GlcNAcylation of adenosine monophosphate-activated protein kinase. Anat Cell Biol. 2018; 51:274–283. PMID: 30637162.13. Balekar N, Katkam NG, Nakpheng T, Jehtae K, Srichana T. Evaluation of the wound healing potential of Wedelia trilobata (L.) leaves. J Ethnopharmacol. 2012; 141:817–824. PMID: 22465731.14. Maioli E, Torricelli C, Fortino V, Carlucci F, Tommassini V, Pacini A. Critical appraisal of the MTT assay in the presence of rottlerin and uncouplers. Biol Proced Online. 2009; 11:227–240. PMID: 19957063.15. Menon MB, Ronkina N, Schwermann J, Kotlyarov A, Gaestel M. Fluorescence-based quantitative scratch wound healing assay demonstrating the role of MAPKAPK-2/3 in fibroblast migration. Cell Motil Cytoskeleton. 2009; 66:1041–1047. PMID: 19743408.16. Ranzato E, Martinotti S, Burlando B. Honey exposure stimulates wound repair of human dermal fibroblasts. Burns Trauma. 2013; 1:32–38. PMID: 27574620.17. Ranzato E, Martinotti S, Burlando B. Epithelial mesenchymal transition traits in honey-driven keratinocyte wound healing: comparison among different honeys. Wound Repair Regen. 2012; 20:778–785. PMID: 22882448.18. Ruthig DJ, Meckling-Gill KA. Both (n-3) and (n-6) fatty acids stimulate wound healing in the rat intestinal epithelial cell line, IEC-6. J Nutr. 1999; 129:1791–1798. PMID: 10498749.19. Calder PC. N-3 polyunsaturated fatty acids, inflammation and immunity: pouring oil on troubled waters or another fishy tale? Nutr Res. 2001; 21:309–341.20. Liu W, Kato M, Akhand AA, Hayakawa A, Takemura M, Yoshida S, Suzuki H, Nakashima I. The herbal medicine shosaiko-to inhibits the growth of malignant melanoma cells by upregulating Fas-mediated apoptosis and arresting cell cycle through downregulation of cyclin dependent kinases. Int J Oncol. 1998; 12:1321–1326. PMID: 9592193.21. Wang ZY, Nixon DW. Licorice and cancer. Nutr Cancer. 2001; 39:1–11. PMID: 11588889.22. Werner S, Krieg T, Smola H. Keratinocyte-fibroblast interactions in wound healing. J Invest Dermatol. 2007; 127:998–1008. PMID: 17435785.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effects of Hydrogen Peroxide on the Migration and Proliferation of the Human Keratinocytes during Wound Healing
- Proliferation of Keratinocytes Induced by Adipose-Derived Stem Cells on a Chitosan Scaffold and Its Role in Wound Healing, a Review
- Corchorus olitorius Ethanolic Extract has Anti-inflammatory and Wound Healing Effects in vitro
- Paracrine Effects of Adipose-Derived Stem Cells on Keratinocytes and Dermal Fibroblasts
- Keratinocyte-Like Cells Trans-Differentiated from Human Adipose-Derived Stem Cells, Facilitate Skin Wound Healing in Mice